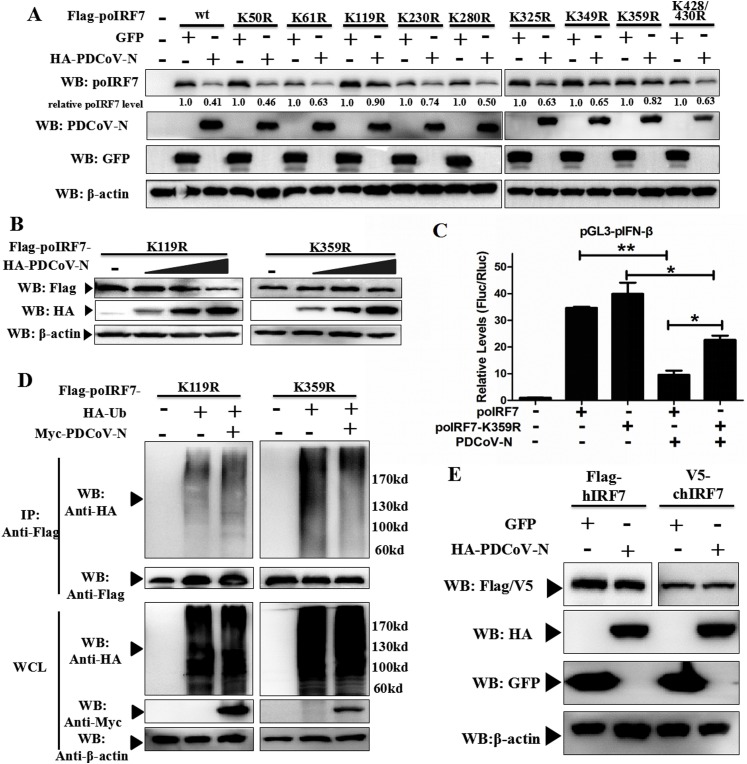
Fig. 7.
PDCoV N protein promoted the ubiquitination of poIRF7 K359 site. (A) PK-15 cells were co-transfected with Flag-tagged wild-type poIRF7 (wt) or its lysine mutants expression plasmids and constructs HA-tagged PDCoV N or GPF protein. Western blot was used to analyze thier expression after 28 h of transfection. The relative expression level of poIRF7 was normalized to the corresponding control group through Image J software analysis. (B) PK-15 cells were co-transfected with Flag-tagged poIRF7 K119R or K359R and dose-dependent HA-tagged PDCoV N protein expression plasmids. (C) HEK293 T cells were co-transfected with HA-tagged Ub, Flag-tagged poIRF7 mutants (K119R, K359R), and Myc-tagged PDCoV N or empty expression plasmids. After 28 h of transfection, the cells were lysed and immunoprecipitated with anti-Flag affinity gel. The WCL and immunoprecipitants were analyzed by Western blot with anti-Flag, anti-HA, anti-Myc and anti-β-actin antibodies. (D) PK-15 cells were co-transfected with Flag-poIRF7 or Flag-poIRF7-K359R or empty vector expression plasmids, along with pGL3-pIFN-β, pRL-TK and HA-PDCoV-N or empty vector expression plasmids. After 24 h transfection, the dual-luciferase assay was performed. All data are presented as means ± SD of three independent experiments (*p < 0.05, **p < 0.01). (E) PK-15 cells were co-transfected with Flag-tagged hIRF7 or V5-tagged chIRF7 and HA-tagged PDCoV N or GFP expression plasmids. At 28 h post-transfection, the cells were lysed to analyze the hIRF7 or chIRF7 expression by Western blot with anti-Flag or anti-V5 antibodies.