ABSTRACT
Cronobacter sakazakii, an emerging opportunistic pathogen, is implicated in severe foodborne outbreak infections in premature and full-term infants. Generally, acid tolerance is vital for the pathogenesis of foodborne pathogens; however, its role in C. sakazakii virulence remains largely unknown. To screen out acid-tolerance determinants from transposon mutants, anovel counterselection method using gentamicin and acid was developed. Using the counterselection method and growth assay, we screened several acid-sensitive mutants and found that nlpD encodes an acid-resistance factor in C. sakazakii.
Compared to the wild-type strain, the nlpD mutant exhibited attenuated virulence in a rat model. Using macrophage THP-1 cells and a pH probe, we verified that nlpD enables bacteria to resist macrophages by resisting acidification. Finally, we confirmed that nlpD maintains C. sakazakii membrane integrity in acid using propidium iodide permeabilization assays via flow cytometry. Our results confirm that nlpD is a novel virulence factor that permits C. sakazakii to survive under acid stress conditions. Considering that NlpD is a conserved lipoprotein located in the bacterial outer membrane, NlpD could be used as a target for drug development.
KEYWORDS: Cronobacter sakazakii, lipoprotein, membrane integrity, acid resistance
Introduction
Cronobacter sakazakii (formerly Enterobacter sakazakii) is an emerging opportunistic foodborne pathogen that causes life-threatening sepsis, meningitis, and necrotizing enterocolitis in premature and full-term infants [1–3]. Since the first report describing an association between C. sakazakii infection and consumption of a powdered infant formula (PIF) in Tennessee in 2001 [4], the risk of infection due to the use of PIF in the neonatal health-care setting has been known. Since then, C. sakazakii has been widely studied around the world.
The virulence traits and pathogenic mechanisms of C. sakazakii remain largely unknown, although initial research has begun to identify specific genes that are involved in the infection process [5–7]. This initial research has revealed that C. sakazakii is able to form biofilms [8], possesses iron acquisition genes [9], and persists in human macrophages [10].
Acid resistance and desiccation resistance are very important for C. sakazakii infection; extraordinary desiccation resistance enables bacteria to survive in PIF, which is the main vehicle of C. sakazakii infection [8]. In addition, C. sakazakii can adapt to a low-pH environment [11]. This characteristic may be associated with the fact that the organism encounters an acidic environment during its passage through the stomach and during its survival within macrophages.
The gastric pH of infants varies from 2.9 to 5.8 after feeding [11,12]. In addition, it has been reported that some organic acids present in the stomach may have antibacterial effects [13]. C. sakazakii can grow at pH values as low as 4.1 [14] and has been shown to tolerate exposure to low pH for long periods [15]. The uptake of invading microorganisms by macrophages is an intrinsic defense response used to kill harmful bacteria. Phagocytes engulf bacteria and form phagolysosomes. The phagolysosomes fuse with lysosomes containing acid hydrolases and free radicals that kill phagocytized bacteria. It has also been reported that acidification may play an important role in killing bacteria [16,17]. C. sakazakii has been shown to survive and even to multiply within macrophages [10,18].
Studies have shown that the acid determinants of bacteria also affect bacterial virulence. For example, deletion of the acid-tolerance response gene dsrA of Salmonella enterica results in defective invasion efficacy and renders the bacterium unable to cause gut inflammation in mice [19]. In addition, the yaeB mutation in Salmonella typhimurium results in increased acid sensitivity and decreased survival within macrophages [20]. Moreover, acid-adapted bacteria demonstrate increased virulence. For example, compared to the nonacid-adapted wild-type Listeria monocytogenes, the acid-adapted strain and a constitutively acid-tolerant mutant showed more invasion of enterocyte-like cells and greater survival within activated macrophages [21]. However, little information is available on the association between acid tolerance and virulence in C. sakazakii.
The objective of this study was to investigate genes putatively associated with acid tolerance-mediated resistance to macrophages and with pathogenicity. We found that nlpD rendered C. sakazakii resistant to acid stress in the stomach and within macrophages by maintaining the bacterial pH. NlpD is a novel virulence factor of C. sakazakii and could be a drug target in the future.
Results
Screening for acid-resistance genes of C. sakazakii
Transposon mutagenesis can efficiently produce a large number of near-random mutations of the bacterial genome; however, subsequent selection of stress-sensitive mutants generally requires a large number of single colony screenings since the rate of transposon insertion is quite low. Here, we developed a counterselection method that relies on the antagonism of an acidic environment to bactericidal antibiotics that specifically kill actively replicating bacteria. Gentamicin is a quick-acting bactericidal antibiotic that is most efficient against rapidly growing bacteria [22]. Therefore, exposure to a moderately acidic pH that inhibits the growth of acid-sensitive mutants should protect these strains from the bactericidal effect of gentamicin, while wild-type strains and mutants that are able to grow under moderately acidic conditions should be killed quickly. Three concentrations of gentamicin were tested for bactericidal activity against C. sakazakii (Fig S1A). Strong bactericidal activity was observed at all three gentamicin concentrations. At 200 mg/L, gentamicin reduced the number of viable C. sakazakii to below the detection level within 60 min. To further select a suitable acidic pH, an LB medium adjusted to various pH values using hydrochloric acid was investigated for its inhibitory effects on the growth of C. sakazakii. The dnaK deletion mutant of C. sakazakii was generated as an acid-sensitive control. Compared to neutral pH, pH 3.0 showed a significant inhibitory effect on the growth of ΔdnaK C. sakazakii compared to its effect on the growth of wild-type C. sakazakii (WT) after culture for 60 min (Fig S1B).
In order to establish a counterselection method that depends on the antagonistic effects of acidic pH on the bactericidal activity of gentamicin, the survival of C. sakazakii was studied after exposure to a combination of acidic pH and gentamicin. The acid-sensitive ΔdnaK mutant strain was capable of withstanding the killing of gentamicin after growth inhibition by acidic pH (Fig S1C). By contrast, rapid killing of the acid-resistant wild-type strain due to gentamicin was observed; the number of viable bacteria of the resistant strain was below the detection threshold after 60 min. Therefore, it appears that the combination of an acidic pH and gentamicin has the desired counterselective effect on acid-sensitive strains of C. sakazakii.
Using this method, we screened several acid-sensitive mutants and identified the insertion sites of their mutations. The phenotype of acid sensitivity was further verified by knocking out the open reading frames of the genes. Finally, we identified five genes, sidA, hmsP, recA, rpfR, and nlpD, that enable C. sakazakii to resist acidic environments (Figure 1) but that do not affect the growth rate of the bacterium in neutral medium (data not shown). NlpD, an extracellular membrane protein, is thought to be related to biofilm formation [23], but it has not previously been reported to be related to acid tolerance. Therefore, the role of nlpD in the acid tolerance and virulence of C. sakazakii was investigated in this study.
Figure 1.
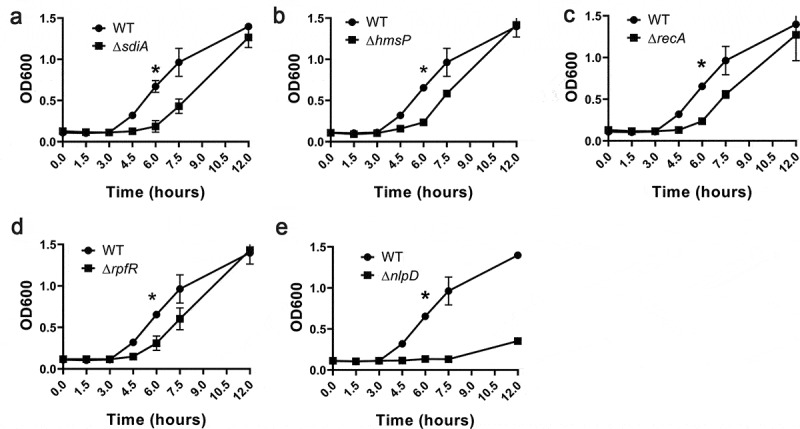
Phenotype verification of five acid determinants using markerless deletion mutants
Growth of (A) wild-type (WT) and ΔsdiA C. sakazakii, (B) WT and ΔhmsP C. sakazakii, (C) WT and ΔrecA C. sakazakii, (D) WT and ΔrpfR C. sakazakii and (E) WT and ΔnlpD C. sakazakii in LB medium at pH 3.0. To initiate experiments, C. sakazakii strains were cultured in Luria-Bertani (LB) overnight at 37°C. In all cases, the estimated initial bacterial densities were 0.1 optical density at 600 nm (OD600). Optical densities of C. sakazakii cultured in LB medium at pH 3.0 were measured immediately before (time zero) and at the indicated time points. Each data point represents the average and standard deviation of three biological repeats.
nlpD knockout reduces bacterial virulence
During the process of C. sakazakii infection, the pathogen is exposed to a variety of acidic environments, including the gastric acid environment, the intestinal acid environment, and the acidic environment inside macrophage phagocytic vesicles. We have verified that nlpD contributes to acid stress induced by hydrochloric acid, which is the main component of gastric acid. However, whether nlpD acts similar under organic acid stress that exists in intestinal and macrophage remains unknown. Therefore, the survival of wildtype, nlpD mutant, and complement strains was tested at low pH adjusted by a diversity of organic acids. Compared with wild-type strain, knockout showed significant growth disadvantages in medium acidified regardless of the pH regulated by acetic acid, butyric acid, or lactic acid (figure S2). These results clearly demonstrated that nlpD confers C. sakazakii with resistance to both inorganic acid stress and organic acid stress.
As an acid-resistance factor, nlpD may affect the pathogenicity of C. sakazakii. Three groups of 3-d-old rats were orally infected with WT, ΔnlpD, and ΔnlpD complemented strains of C. sakazakii, and the survival of the animals was monitored for more than 5 d. Rats infected with WT bacteria or with strains in which the mutations were complemented began to die at 12 hours, and 80% of the animals had died by 72 hours. In contrast, rats infected with ΔnlpD bacteria began to die at 48 hours, and 80% of them survived for at least 72 hours after infection. The significant difference between the ΔnlpD and WT strains was identified by a survival curve (Figure 2a). Whether nlpD affects the colonization of C. sakazakii in mice was also investigated. The bacterial load of ΔnlpD bacteria in blood (Figure 2b), liver (Figure 2c), and spleen (Figure 2d) was 8- to 20-fold lower than that of WT bacteria. In addition, intestinal tissue from rats infected with the WT strain showed dilation and necrosis by 48 h post-infection, accompanied by perforation and destruction of villi, whereas intestinal tissue damage was significantly less severe in ΔnlpD-infected rats (Figure 2e and figure 2f). These results indicate that nlpD knockout decreases the virulence of C. sakazakii.
Figure 2.
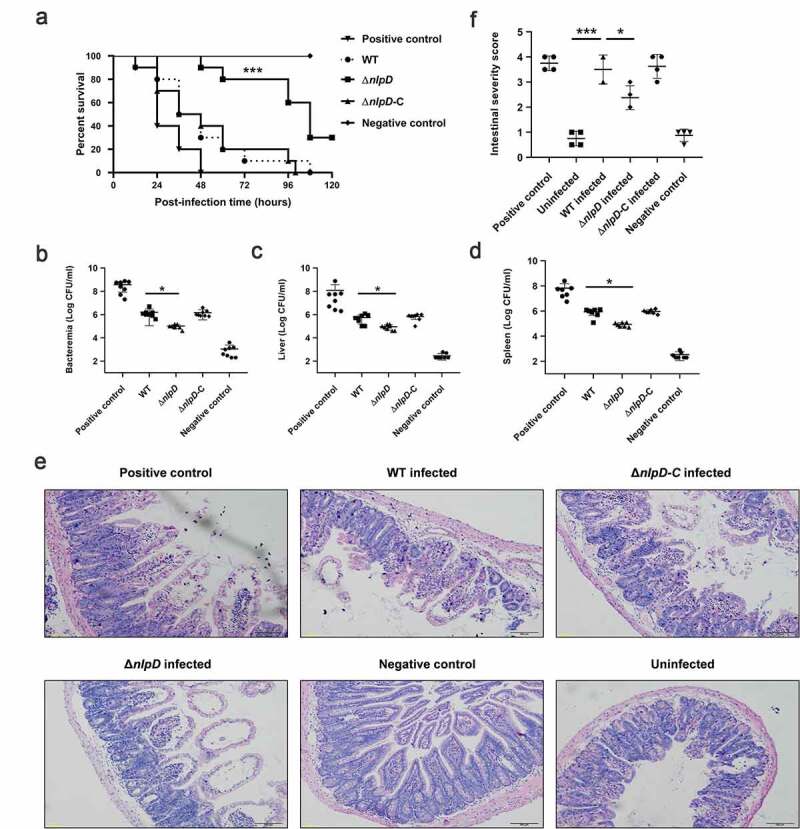
The acid determinant nlpD is involved in the pathogenicity of Cronobacter sakazakii.
(A) Survival of rat pups during the 120-hour period following oral infection with various bacterial strains. Combined data from three independent experiments are shown (log-rank test; ***, P < 0.001). (B–D) Colony-forming units (CFU) present in the blood, liver, and spleen of infected rats at 24 hours post-infection. Blood was collected from the facial vein, and the liver and spleen were harvested and homogenized in ice-cold PBS for use in colony enumeration. (E) Histopathologic examination of intestinal tissues of uninfected, WT-infected, and ΔnlpD-infected rats. Scale bars, 100 μm. (F) Intestinal sections obtained at 24 hours post-infection were graded as grade 0 (normal) to grade 4 (severe) by a pathologist blinded to the groups according to morphological changes (n = 4). Salmonella enterica serovar Typhimurium and E. coli DH5α serve as positive and negative controls, respectively.
nlpD does not affect bacterial adhesion
NlpD is a lipoprotein that is thought to be involved in biofilm formation [24]. We next tested the effect of nlpD on bacterial adhesion. A gentamicin protection test was conducted to study the role of nlpD in colonization by C. sakazakii of the small intestinal epithelial cell line Caco-2 and the human brain microvascular endothelial cell line HBMEC. No significant differences in colonization by WT, ΔnlpD, or ΔnlpD-C C. sakazakii were observed in either of these cell lines (Figure 3). Therefore, it is clear that nlpD does not affect colonization by C. sakazakii in vitro.
Figure 3.
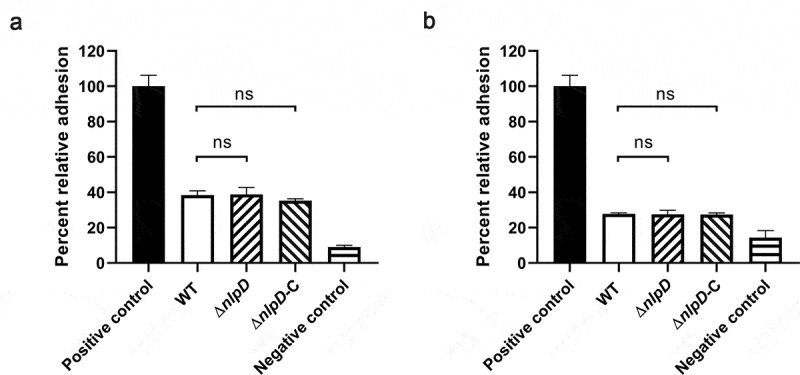
nlpD is not involved in adherence
(A, B) Relative adhesion of WT, ΔnlpD mutant, and complemented strain to Caco-2 (A) and human brain microvascular endothelial cells (HBMECs) (B). The data are presented as the mean and standard deviation of three biological repeats; n = 3 (Student’s t-test; ns, no statistically significant difference) of three biological repeats. To initiate experiments, C. sakazakii Salmonella enterica serovar Typhimurium and DH5α were cultured in LB overnight at 37°C. Listeria monocytogenes EGD-e was cultured in BHI overnight at 37°C. Salmonella enterica serovar Typhimurium was included as a positive control in adhesion to Caco-2 (A). Positive control in adhesion to HBMECs was Listeria monocytogenes EGD-e (B). E. coli DH5α served as a negative control in adhesion to both Caco-2 and HBMECs.
C. sakazakii requires nlpD for survival in macrophages
Previous studies have found that C. sakazakii resists killing by macrophages [18,25]. Phagocytes mainly use acidic environments, ROS, and lysozyme to kill intracellular bacteria. Based on our discovery that nlpD is an acid-resistance factor in C. sakazakii, nlpD may be involved in the tolerance of this bacterium to killing by macrophages. To determine whether nlpD affects the macrophage tolerance of C. sakazakii, non-activated and IFN-γ-activated macrophages derived from human THP-1 cell lines were challenged with WT, ΔnlpD, or ΔnlpD-C strains. All of the strains survived and replicated in non-activated macrophages (Figure 4a). In IFN-γ-activated macrophages, the CFU of the WT and nlpD-C strains remained basically unchanged after the addition of gentamicin (Figure 4b); however, the number of viable ΔnlpD bacteria showed a continuous decline after the addition of gentamicin, and after 8 hours, the CFU of ΔnlpD bacteria was below the detection threshold. Thus, the ΔnlpD strain did not resist killing by macrophages; therefore, C. sakazakii needs nlpD to survive in macrophages.
Figure 4.
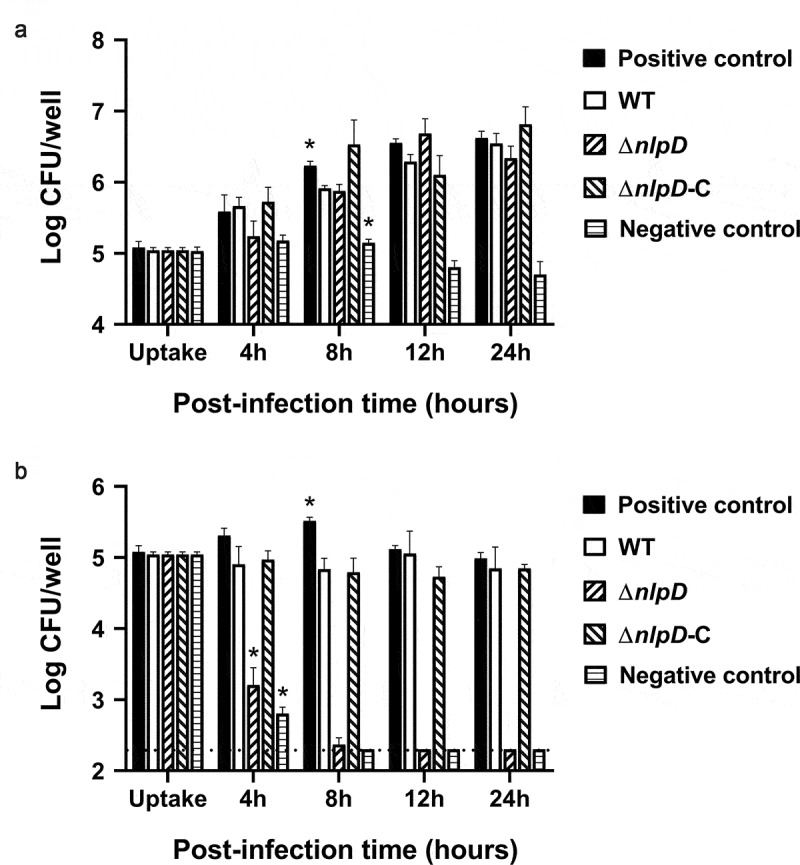
The ΔnlpD mutant shows attenuated survival within activated macrophages
(A, B) Survival of WT, ΔnlpD, and complemented strains within nonactivated macrophages (A) and IFN-γ-activated macrophages. (B) The cells were harvested and resuspended in 1% Triton X-100 buffer to separate aggregates of bacteria within the cells at the indicated time points. THP-1 cells were differentiated into macrophages using phorbol 12-myristate 13-acetate treatment at 60 ng/mL for 48 hours, and interferon-γ (IFN-γ) was used to activate macrophages for enhanced microbial killing. C. sakazakii Salmonella enterica serovar Typhimurium and DH5α were cultured in LB overnight at 37°C. Strains were added to THP-1 cells at an MOI of 100. The bacteria were plated on LB agar, and CFU was counted after culture for 24 hours. The assays were repeated three times biologically, and the data represent the average and standard deviation. Salmonella enterica serovar Typhimurium and DH5α served as positive and negative controls, respectively. *Comparison of indicated strain with WT yielded p < .05 with one-way ANOVA.
Cronobacter sakazakii requires nlpD to maintain its pH in macrophages
To determine whether the reduced macrophage survival of nlpD was due to the acidic environment inside the macrophage, the pHluorin2 gene, which encodes an enhanced, ratiometric, pH-sensitive green fluorescent protein, was cloned into the pUC57 vector and transformed into C. sakazakii. Fluorescence images were generated using a confocal microscope, and the pH of the bacterial cells was analyzed. In IFN-γ-activated macrophages, the pH of the WT strain was observed to be above 6, while that of the ΔnlpD strain was below 5 (Figure 5a and 5b). Therefore, the presence of nlpD maintains the internal pH of the bacteria in macrophages. However, it is surprising that a pH equal to that within macrophages does not kill the ΔnlpD strain. ΔnlpD still grew at a pH of 4.5, similar to the pH within lysosomes, although it showed slower growth than the WT strain (Figure 5c). Therefore, the hypothesis that the synergistic action of low pH and other factors present in macrophages can kill ΔnlpD was proposed. The synergistic effect of pH 4.5 and ROS was verified by the marked reduction in the in vitro survival of the ΔnlpD mutant in H2O2 at pH 4.5 (Figure 5e); this reduction in survival did not occur when the mutant was exposed to H2O2 at a neutral pH (Figure 5d). These results clearly indicate that nlpD indeed facilitates resistance to the synergistic effect of bactericidal acidity and reactive oxygen species in macrophages.
Figure 5.
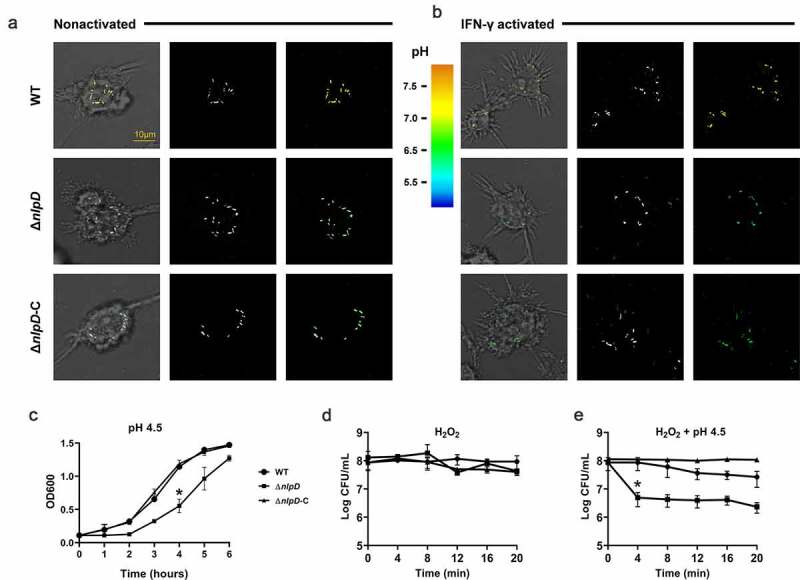
nlpD deletion attenuates bacterial survival within IFN-γ-activated macrophages
Transmitted images with overlays of bacteria in green (left), fluorescent bacteria (center), and pseudocolor images of the 410:470 excitation ratio (right) of nonactivated (A) and IFN-γ–activated (B) macrophages 24 h after infection with WT, the ΔnlpD mutant, and the complemented mutant are shown. Scale bar, 10 μm. (C) Sensitivities of C. sakazakii strains to pH 4.5. (D) Sensitivities of C. sakazakii strains to 200 mM hydrogen peroxide (H2O2). (E) Sensitivities of C. sakazakii strains to a combination of 200 mM hydrogen peroxide (H2O2) and pH 4.5. To initiate experiments, C. sakazakii strains were cultured in Luria-Bertani (LB) overnight at 37°C. In all cases, the estimated initial bacterial densities were 0.1 optical density at 600 nm. Optical densities or CFU counts of C. sakazakii were determined immediately before (time zero) and at the indicated time points. All data represent the mean and standard deviation of three biological repeats.
nlpD activation depends on EnvZ
The EnvZ/OmpR two‐component system has been reported to be involved in signal transduction during acid stress [26,27]. In C. sakazakii, we also found that ΔevnZ and ΔevnZΔompR showed attenuated acid resistance compared to WT (Figure 6a and 6b). Considering that NlpD is a lipoprotein located in the outer membrane and that the two-component system can widely activate the expression of outer membrane proteins [28–30], we speculated that the EnvZ/OmpR two‐component system might affect the expression of nlpD. To determine whether the absence of EnvZ affects the expression of nlpD, RT-qPCR was used to measure the level of transcription of nlpD in the WT and ΔevnZ strains. In neutral medium, ΔevnZ showed a similar level of nlpD transcription compared to the WT strain (Figure 6c). In the acidic environment, a significant reduction in nlpD transcription in ΔevnZ was observed compared with the WT strain (Figure 6d), suggesting that nlpD transcription is regulated by EnvZ. At the same time, the results showed that nlpD transcription was significantly higher in an acidic pH environment than at neutral pH, suggesting that nlpD transcription is activated by acidic environments (Figure 6e). In addition, western blotting was used to directly detect the expression of His6-tagged NlpD. The NlpD level in ΔevnZ was significantly lower than that in the WT strain under acidic conditions, but no difference was observed at neutral pH (figure 6f). These results indicate that the activation of nlpD in acidic environments is dependent on the presence of EnvZ.
Figure 6.
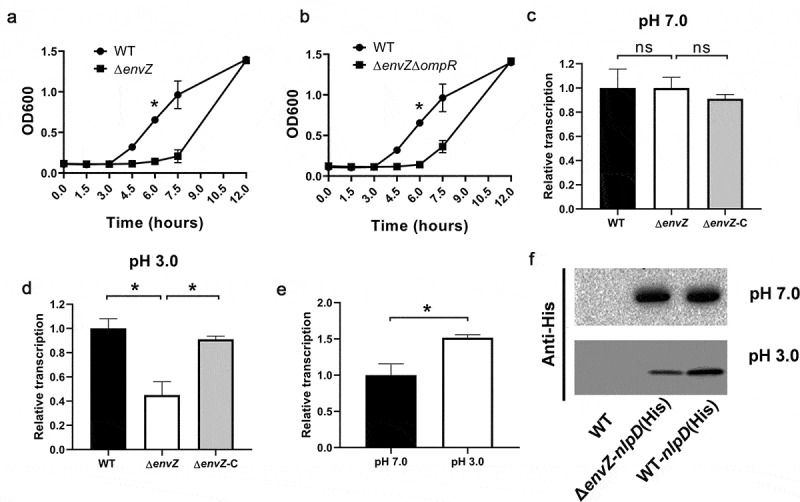
Reduced nlpD expression in the ΔevnZ mutant
(A, B) Growth curves of wild-type (WT), ΔenvZ (A), and ΔenvZΔompR (B) strains in LB medium at pH 3.0. To initiate experiments, C. sakazakii strains were cultured in Luria-Bertani (LB) overnight at 37°C. In all cases, the estimated initial bacterial densities were 0.1 optical density at 600 nm. Optical densities of C. sakazakii were determined immediately before (time zero) and at the indicated time points. (C, D) Relative transcription of nlpD in WT, ΔevnZ, and the ΔevnZ complemented strain at neutral pH (C) and at pH 3.0 (D). (E) Relative transcription of nlpD in the WT strain at neutral pH and at pH 3.0. (F) Western blotting analysis of the levels of His6-tagged NlpD in WT, ΔevnZ-nlpD (His), and WT-nlpD (His) at neutral pH and at pH 3.0. All data represent the average and standard deviation of three biological repeats performed.
nlpD maintains membrane integrity in acid
Next, we explored the molecular mechanisms responsible for the function of nlpD that were observed above. We have disclosed that in addition to nlpD, four other genes rpfR, hmsP, sdiA, and recA also conferred C. sakazakii acid resistance (Figure 1). Moreover, acid environment could promote the transcription of hmsP, sdiA, and recA in C. sakazakii (Fig S3 B-D). However, the transcription of rpfR, hmsP, sdiA, and recA was hardly changed in nlpD-knockouted strain in neither neutral pH nor pH 3.0 (Fig S3), suggesting nlpD provides acid resistance but independent of rpfR, hmsP, sdiA, and recA.
A low-pH environment can affect the function of proteins, including the proteins of intermediate metabolism and the bacterial envelope. Previous studies have shown that a low pH can affect the function of the bacterial membrane, including increasing the sensitivity of bacteria to hydrophobic drugs, the loss of mobility, and reducing the ability to form biofilm [31], suggesting that a low pH can cause bacterial membrane damage. As a membrane protein, we speculate that nlpD could maintain the membrane integrity of C. sakazakii at a low pH. Consistent with this conjecture, we observed an increase in the permeability of propidium iodide to C. sakazakii at pH 3.0 compared with pH 7.0 (Figure 7a). However, the nlpD mutant significantly increased the permeability of propidium iodide in an acidic environment (Figure 7b), suggesting that nlpD could maintain the integrity of the bacterial cell membrane in an acidic environment.
Figure 7.
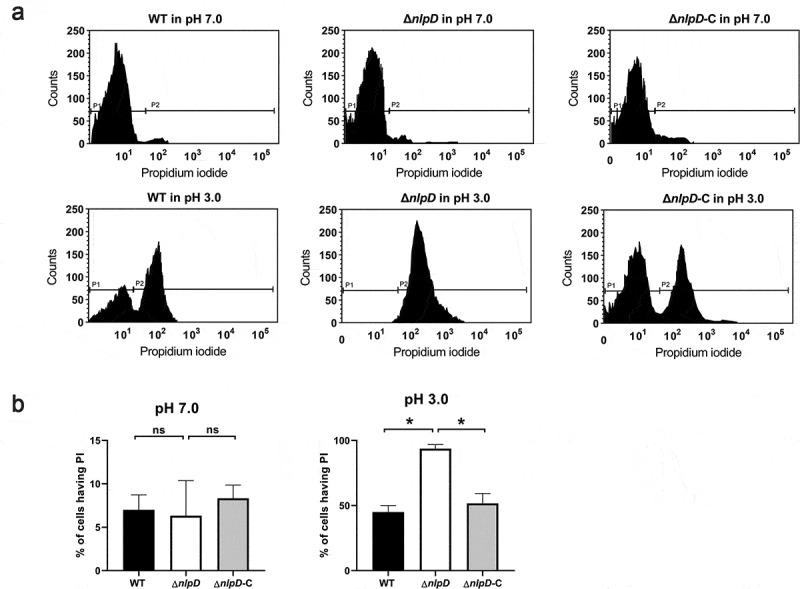
Membrane permeabilization of C. sakazakii by PI uptake assay
(A) Membrane permeabilization of C. sakazakii at pH 7.0 or pH 3.0. Logarithmic C. sakazakii BAA894 and its ΔnlpD mutant were analyzed for membrane permeabilization after incubation in pH 3.0 or pH 7.0 for 2 hours. A total of 10,000 cells were acquired for each flow cytometry analysis. (B) Histograms showing the percent of cells having PI based on data obtained by flow cytometry assays described in Figure 7a of three independent experiments. These data represent the mean (±SD) of three independent experiments (*P ≤ 0.05).
Discussion
The low pH of gastric secretions is considered the body’s first line of defense against food-borne pathogens. The ability of bacteria to resist being killed by acid during passage through the stomach increases their likelihood of colonizing the intestines and causing an infection [32]. Thus, foodborne pathogens have evolved a variety of acid-tolerance genes for survival in these acidic environments [33–36]. Although many studies have identified a series of acid determinants in vitro, only a few recent reports have linked bacterial acid-tolerance genes to bacterial virulence. In our work, we found that the acid-tolerance gene nlpd of C. sakazakii confers tolerance to macrophages and is a virulence factor. Our study suggests that more attention should be paid to the search for virulence factors from the perspective of acid-tolerance genes.
Our study demonstrated for the first time that C. sakazakii can grow at pH 3.0, while previous investigations have found that C. sakazakii can withstand an acidity of pH 3.5 [37]. In fact, C. sakazakii exists widely in the environment and in food, where the environmental pH changes over time [38]. Therefore, it is not surprising that C. sakazakii has extraordinary acid tolerance.
NlpD was found to confer macrophage tolerance as well as tolerance of low pH. Previous studies have found that macrophage removal aggravates C. sakazakii infection [25], suggesting that macrophages provide protection against C. sakazakii infection in mice. Moreover, we found that macrophages can kill nlpD-knockout bacteria efficiently, indicating that these cells do indeed play a bactericidal role, but nlpD provides macrophage tolerance. Therefore, the macrophage tolerance of C. sakazakii has an important impact on the pathogenicity of the bacterium.
Macrophages rely on acidity, ROS, and other substances to kill intracellular bacteria [39]. Bacteria have evolved multiple mechanisms to resist killing by macrophages. For example, the molecular chaperone DnaK can repair proteins that have been damaged by macrophages and contributes to the survival of Salmonella within macrophages [40]. The efflux pump EmrKY contributes to the survival of Shigella within macrophages [41]. In addition, a family of surface-exposed virulence factors termed “macrophage infectivity potentiators” (MIPs) have been described in intracellular microorganisms, and these virulence factors are necessary for the survival of Neisseria gonorrhoeae [42] and Legionella pneumophila [43] within macrophages. Recently, the mip-like gene fkpA of C. sakazakii has been shown to play a role in macrophage resistance, but its role in virulence has not been elucidated [10]. Previous studies have mostly considered that nlpD is related to envelop integrity and iron acquisition [44–46]. However, a few studies reported that nlpD affects virulence in the phytopathogens Xanthomonas [47] and Yersinia pestis [48]. Except in Yersinia pestis, however, the virulence mechanism of nlpD remains poorly understood. Our results reveal for the first time the relationship between nlpD and acid resistance and demonstrate that nlpD leads to macrophage tolerance and virulence based on an acid-tolerance mechanism.
Similar to the observation in Vibrio cholerae, Salmonella dublin, and Escherichia coli, rpoS in C. sakazakii was identified to locate downstream of nlpD as well. rpoS has been verified to be associated with the resistance to various environmental stresses, such as oxidative stress, carbon starvation, UV irradiation, and acidic conditions. The adjacent location of nlpD and rpoS clusters potentially indicated the synergistic effect on environmental stress responses. However, the stop codon introduced by the point mutation C994A in rpoS of C. sakazakii leads to a premature termination of the sequential translation. The defect of rpoS in C. sakazakii could potentially pose an emphatic participation of nlpD in bacterial acid resistance and pathogenicity.
Two-component systems are widely used by bacteria to sense and respond to environmental changes. We found that C. sakazakii can regulate the expression of the acid stress gene nlpD through the EnvZ-OmpR two-component system. Similarly, the acid response in Group B Streptococci is regulated by the CovS/CovR two-component system [49]. In fact, EnvZ-OmpR is a two-component system that exists widely in bacteria and is believed to be related to biofilm formation and osmotic tolerance [50–52]. EnvZ has also been widely reported to regulate the expression of a variety of outer membrane proteins [53–55]. Our experiments demonstrate that EnvZ can sense external acidic environments and can regulate the level of the outer membrane protein NlpD, thereby affecting the acidic tolerance of bacteria.
NlpD is a conserved protein present in bacteria that plays an important role in pathogenicity and may serve as a target for future drug development.
Materials and methods
Bacterial strains, plasmids, and growth conditions
The strains and plasmids used in this study are listed in Table 1, and the primers are listed in Table 2. Bacteria were stored in LB broth containing 15% glycerol (Biosharp, China) at −80°C. To initiate all experiments, strains were revived in LB broth (Oxoid, UK). When necessary, antibiotics were added at final concentrations of 100 μg mL−1 ampicillin (Sangon, China) or 50 μg mL−1 kanamycin (Sangon, China). E. coli DH5α (Weidi, China) was used as the host for the preparation of plasmid DNA, and E. coli S17 lambda pir (Weidi, China) was used for preparation of the pCVD442 suicide vector [56]. Plasmid construction was performed according to standard protocols with the minor modification that cloning of PCR fragment into the linearized vector was accomplished using a commercial seamless cloning and assembly kit (Vazyme, China). pCVD442 (Miaolingbio, China) was linearized by PCR using the primer pair pCVD442-fwd and pCVD442-rev, and the upstream and downstream fragments of genes to be knocked out were amplified by PCR using the primers listed in Table 2. In-frame deletion mutants were generated using the pCVD442 suicide vector method described previously [57]. Deletion mutants were complemented using the low-copy vector pACYC184 (Miaolingbio, China). ΔnlpD complementation was performed using the pACYC184 plasmid containing an nlpD gene fragment amplified using the primer pair nlpD-comp-fwd and nlpD-comp-rev. nlpD(his) complementation in the ΔnlpD mutant was performed using the pACYC184 plasmid containing an nlpD(his) gene fragment amplified using the primer pair nlpD(his)-fwd and nlpD(his)-rev. Transformation and selection of C. sakazakii were performed using the method described previously.
Table 1.
Bacterial strains and plasmids used in this study
| Strains, plasmids | Description | Reference, source |
|---|---|---|
| Cronobacter sakazakii | ||
| WT | Wild-type Cronobacter sakazakii BAA-894 | [64] |
| ΔdnaK | Markerless deletion mutant ΔdnaK | This study |
| ΔsidA | Markerless deletion mutant ΔsidA | This study |
| ΔhmsP | Markerless deletion mutant ΔhmsP | This study |
| ΔrecA | Markerless deletion mutant ΔrecA | This study |
| ΔrpfR | Markerless deletion mutant ΔrpfR | This study |
| ΔnlpD | Markerless deletion mutant ΔnlpD | This study |
| ΔenvZ | Markerless deletion mutant ΔenvZ | This study |
| ΔenvZΔompR | Markerless deletion mutant ΔenvZΔompR | This study |
| ΔnlpD-C | nlpD complementation in ΔnlpD | This study |
| ΔnlpD-nlpD(his) | nlpD(his) complementation in ΔnlpD | This study |
| ΔenvZ-nlpD(his) | nlpD(his) complementation in ΔenvZ | This study |
| WT-nlpD(his) | nlpD(his) complementation in WT | This study |
| WT-pHluorin2 | WT harboring pACYC184-pHluorin2 | This study |
| ΔnlpD-pHluorin2 | ΔnlpD harboring pACYC184-pHluorin2 | This study |
| ΔnlpD-C-pHluorin2 | ΔnlpD-C harboring pACYC184-pHluorin2 | This study |
| Escherichia coli | ||
| E. coli DH5α | Strain for construction and controls | [22] |
| S17 lambda pir | Strain for construction harboring lambda pir | [65] |
| S17 lambda pir-ΔdnaK | S17 lambda pir harboring pCVD442-ΔdnaK | This study |
| S17 lambda pir-ΔsidA | S17 lambda pir harboring pCVD442-ΔsidA | This study |
| S17 lambda pir-ΔsidA | S17 lambda pir harboring pCVD442-ΔsidA | This study |
| S17 lambda pir-ΔsidA | S17 lambda pir harboring pCVD442-ΔsidA | This study |
| S17 lambda pir-ΔrpfR | S17 lambda pir harboring pCVD442-ΔrpfR | This study |
| S17 lambda pir-ΔnlpD | S17 lambda pir harboring pCVD442-ΔnlpD | This study |
| S17 lambda pir-ΔenvZ | S17 lambda pir harboring pCVD442-ΔenvZ | This study |
| S17 lambda pir-ΔenvZΔompR | S17 lambda pir harboring pCVD442-ΔenvZΔompR | This study |
| Salmonella enterica | ||
| Salmonella enterica serovar Typhimurium | Standard/reference strain used as control | [66] |
| Listeria monocytogenes | ||
| Listeria monocytogenes EGD-e | Standard/reference strain used as control | [67] |
| Plasmids | ||
| pACYC184 | Low-copy plasmid | [64] |
| pACYC184-nlpD | nlpD complementation vector | This study |
| pACYC184-nlpD(his) | vector harboring nlpD fused with His tag | This study |
| pACYC184-pHluorin2 | pACYC184 harboring pHluorin2 | This study |
| pCVD442 | Suicide plasmid for markerless deletion | [68] |
| pCVD442-ΔdnaK | dnaK deletion plasmid | This study |
| pCVD442-ΔsidA | sidA deletion plasmid | This study |
| pCVD442-ΔhmsP | hmsP deletion plasmid | This study |
| pCVD442-ΔrecA | recA deletion plasmid | This study |
| pCVD442-ΔrpfR | rpfR deletion plasmid | This study |
| pCVD442-ΔnlpD | nlpD deletion plasmid | This study |
| pCVD442-ΔenvZ | envZ deletion plasmid | This study |
| pCVD442-ΔenvZΔompR | envZ-ompR deletion plasmid | This study |
Table 2.
Primers used in this study
| Primers | Sequence (5′-3′) | ||||
|---|---|---|---|---|---|
| For Construction | |||||
| pCVD442-fwd | GGCTGTCAGACCAAGTTTACTCATATATACTTTAGATTG | ||||
| pCVD442-rev | GCAGATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTG | ||||
| ΔdnaK-A | GAAAAAGGAAGAGTATCTGCGGTACGGTACGGCCATACTGTTCG | ||||
| ΔdnaK-B | GCGATTAACCCATCTAAACGTCTCCACTAAAAAATCGTCATC | ||||
| ΔdnaK-C | CGTTTAGATGGGTTAATCGCCCTGATGCAGGGTAGATAAC | ||||
| ΔdnaK-D | GATTAATTGTCAAGGCTAGCGTGCTGTTTCACCTGCACCTGAAC | ||||
| ΔsidA-A | GAAAAAGGAAGAGTATCTGCGAACCACGCTTTTGCGTAGTATTAAC | ||||
| ΔsidA-B | CAAATCAGTCCAATGTCACTTTCCTTCATGCTGTG | ||||
| ΔsidA-C | GTGACATTGGACTGATTTGAGCGGGTCATTTGTTC | ||||
| ΔsidA-D | GATTAATTGTCAAGGCTAGCATCTTCAAGTATGCGTCGTATC | ||||
| ΔhmsP-A | GAAAAAGGAAGAGTATCTGCGTGGATGAACGGCTATTACTAC | ||||
| ΔhmsP-B | CGAAACCATCGCCATCTGTTTTATCGTGAGGGAAC | ||||
| ΔhmsP-C | AGATGGCGATGGTTTCGGCCGTTTCCCCGTGACGTCGCTGCAAAAGGTGTAAG | ||||
| ΔhmsP-D | GATTAATTGTCAAGGCTAGCTACCGATGGTGAACGCCATC | ||||
| ΔrecA-A | GAAAAAGGAAGAGTATCTGCGTTGGCGATCACACCGAAGCAAG | ||||
| ΔrecA-B | TCTTCGTTGGTCTGCTTGTTTTCGTCGATAGCCATTTTTAC | ||||
| ΔrecA-C | ACAAGCAGACCAACGAAGAGTTTTAATCTCAAG | ||||
| ΔrecA-D | GATTAATTGTCAAGGCTAGCCACGTCCTTGAACTGGTTCATC | ||||
| ΔrpfR-A | GAAAAAGGAAGAGTATCTGCGATGGTGGGTCAACAATCAATG | ||||
| ΔrpfR-B | AGGCATTCGTCGTCATCACAACC | ||||
| ΔrpfR-C | GTGATGACGACGAATGCCTGAGCTGCAATCACGTC | ||||
| ΔrpfR-D | GATTAATTGTCAAGGCTAGCAGCAGCTTCAGGCACGCAAG | ||||
| ΔnlpD-A | GAAAAAGGAAGAGTATCTGCGTTCTGAATCAACTGCGTGCTCAGG | ||||
| ΔnlpD-B | GCACTCTGCCAATTTATCGCTGCGATGGCGGCATAATCATAATCATCC | ||||
| ΔnlpD-C | CGATAAATTGGCAGAGTGCGCTTCTTCAG | ||||
| ΔnlpD-D | GATTAATTGTCAAGGCTAGCGCAGACCGAAACGACGGGCCAGAAC | ||||
| ΔenvZ-A | GAAAAAGGAAGAGTATCTGCGGTTTTGTGATGAAAAGTGAAG | ||||
| ΔenvZ-B | CTAGCTGTTCGAGAAGCGCAGCTTCCTCATG | ||||
| ΔenvZ-C | GCTTCTCGAACAGCTAGTTTTCTGTTCACGCCATC | ||||
| ΔenvZ-D | GATTAATTGTCAAGGCTAGCGATGACGGCGTATTTAACTTC | ||||
| ΔenvZΔompR-A | GAAAAAGGAAGAGTATCTGCGTCATTCATCATGGCAATATCATC | ||||
| ΔenvZΔompR-B | CCGTAGGCTCAAACAGTTGTAACGCCATTC | ||||
| ΔenvZΔompR-C | AACTGTTTGAGCCTACGGTAGTTAAAAACAGCTAGTTTTCTG | ||||
| ΔenvZΔompR-D | GATTAATTGTCAAGGCTAGCCCGAACCGGATATCTATCATG | ||||
| nlpD-comp-fwd | GGTCTAGATAGAGAAGTAAACCATTCCAG | ||||
| nlpD-comp-fwd | GGTCTAGATCTTCATTTAAGTCATGAAC | ||||
| nlpD(his)-fwd | GGTCTAGATAGAGAAGTAAACCATTCCAG | ||||
| nlpD(his)-rev | GGTCTAGATTAATGATGATGATGATGATGTCGCTGCGGCAAATAGC | ||||
| For sequencing confirmation | |||||
| ΔdnaK-E | GTGGCACGACGTCGGGTAAATC | ||||
| ΔdnaK-F | GTGGCCGCCATCGCAAAGTTGATC | ||||
| ΔsidA-E | GTGGTGCAGACCGGAGAAGTGGTC | ||||
| ΔsidA-F | GTGGCACATCCACCGGGTGAATGCG | ||||
| ΔhmsP-E | GTGGTCTTAACGCCGATCAGCTCAC | ||||
| ΔhmsP-F | GTAGAAGCAGACAATCAGCTG | ||||
| ΔrecA-E | GTGGAATACAGCGTCGGCCAGCTG | ||||
| ΔrecA-F | GTGGTTCCAGGTCGTTGTGCTTAC | ||||
| ΔrpfR-E | GTGGCTTCCAGTCGTTTGCGGAAATC | ||||
| ΔrpfR-F | GTGGATACAGCGCGTTTTACTCAAC | ||||
| ΔnlpD-E | GTGGAGTGAACGGGCAATGGTAAAC | ||||
| ΔnlpD-F | GTGGACATCTTCAAGCGTTGCC | ||||
| ΔenvZ-E | GTGGTTGGCAGCGTCGTCATATCG | ||||
| ΔenvZ-F | GTGGCACCGGCAAAACGACGCTTTC | ||||
| ΔenvZΔompR-E | GTGGATCAAGCAGGCGAATGGCAG | ||||
| ΔenvZΔompR-F | GTGGCACCGGCAAAACGACGCTTTC | ||||
| For RT-PCR | |||||
|
nlpD RT-F nlpD RT-R hmsP-RT-F hmsP-RT-R sdiA-RT-F sdiA-RT-R recA-RT-F recA-RT-R rpfR-RT-F rpfR-RT-R 16s rRNA-F 16s rRNA-R |
GTTTACAATCGCAAGTATG CCAGGCGATATAAAACAG CGATCACCTGGTGCATCAAC TGCAGACGCGGAATAGTGAG TATTTCGCGCTGTGCGTTCG TCCGCCTGGTAATGCTCAAG GGTTGATCTTGGCGTGAAGC GAGGAAGTTGGTCGCATTCG ATCGCCACGGCAACATTCAG CTTCAGCGGGCGTCATAAAG GCCTCATGCCATCAGATGTG TCTGGACCGTGTCTCAGTTC |
||||
Random mutagenesis and screening
The transposon mutagenesis in the strain C. sakazakii was performed according to protocols described previously [58]. Shortly, a transposon insertion library was constructed by using the EZ-Tn5< KAN-2> Tnp Transposome kit (NovoBiotec, China) according to the manufacturer’s instruction. To select for transposon insertion clones, 100 μl aliquots were plated onto LB agar plates containing 50 μg/ml kanamycin, and plates were incubated for 24 h at 37°C. Afterward, single colonies were rinsed three times with PBS. The bacterial suspension was centrifugated at 12,000 rcf for 1 minute. The supernatant was discarded, and the residue was washed twice with LB broth (pH 3.0). Aliquots (1 mL) were diluted 10-fold in LB broth (pH 3.0). After half an hour, 5-mL aliquots of the dilution were subcultured in 5 mL LB broth (pH 3.0) containing 100 mg/L gentamicin at 37°C with shaking for 1 hour. The strains were then serially diluted and plated on LB agar (pH 7.0). The colonies growing on LB agar were further tested in acidic challenge assays to determine their acid sensitivity. The randomness of insertions was verified according to the previously described method [59]. Genomic DNA was analyzed from individual mutants by Southern blotting using a digoxigenin probe against the kanamycin-resistance cassette contained within the transposon to confirm single transposon insertions.
Bacterial survival assays
The bacterial survival assays were performed according to protocols described previously with small changes [60]. In the activation assays, 200 mM H2O2 and pH 3.0 were used.
Macrophage survival assay
Macrophage survival was analyzed as described previously with minor changes [10], namely, that THP-1 cells were differentiated into macrophages using phorbol 12-myristate 13-acetate (Aladdin, China) (PMA) treatment at 60 ng/mL for 48 hours, and interferon-γ (ProteinTech, USA) (IFN-γ) was used to activate macrophages for enhanced microbial killing. C. sakazakii strains were added to THP-1 cells at an MOI of 100. Salmonella enterica serovar Typhimurium and DH5α at the same MOI were used as positive and negative controls, respectively.
Intracellular pH measurement
Intracellular pH measurement assays were performed as described previously [61], except that the gene pHluorin2, which encodes an enhanced, ratiometric, pH-sensitive green fluorescent protein, was used in the pACYC184 vector and transformed it into C. sakazakii. Images were acquired using a Leica SPE confocal microscope (Leica, Germany) with dual excitation at 405 nm and 485 nm and an emission filter of 535 nm.
RT-PCR
RT-PCR was performed in a Bio-Rad CFXConnect™ system (Bio-Rad, USA) using SYBR® green mix (NEB, USA) according to standard methods. The level of transcription was assessed through qPCR and normalized with internal control 16s rRNA using specific primers listed in Table 2. Thermocycling conditions for qPCR were as follows: initial denaturation at 92°C for 3 min and then denaturation at 92°C for 5 s, annealing at 56°C for 5 s, extension at 72°C for 5 s, and melt curve analysis at 65–95°C. Amplification of the single PCR product was confirmed by monitoring the dissociation curve followed by melting curve analysis.
Rat virulence assay
Rat infections were conducted as described previously with minor changes [62]. Salmonella enterica serovar Typhimurium and E. coli DH5α served as positive and negative controls, respectively. Uninfected group was treated with a single dose of LB (100 μL) and infected groups with bacterial culture (107 CFU) by oral gavage. Then, the animals were maintained atypical condition with mother. The survival of the infected animals was recorded at defined time intervals. To analyze bacterial colonization of organs, the rats were sacrificed 24 hours after infection. Organs were homogenized in PBS, the homogenates were serially diluted, and the number of bacteria was determined by plating the dilutions on LB agar.
Adhesion assay
Bacterial adhesion was measured as described previously with small modifications [63]. C. sakazakii was prepared and applied to the Caco-2 cell monolayer at an MOI of 100. After 45 min incubation, the plates were washed three times with PBS, and the cells were lysed in 1% Triton X-100 (Abcone, China). The cell suspensions were serially diluted and plated on LB agar for enumeration of adherent bacteria. Salmonella enterica serovar Typhimurium was included as a positive control for adhesion to Caco-2. Listeria monocytogenes EGD-e was used as a positive control for adhesion to HBMECs. E. coli DH5α served as a negative control for adhesion to both Caco-2 and HBMECs.
Immunoblot analysis of His6-tagged proteins
Immunoblot analysis was performed as described previously with small modifications [31]. Briefly, bacterial samples were disrupted by sonication (TissueLyser, China). The cell lysates were subsequently centrifuged, and the precipitates were transferred to membrane protein dissolution buffer (Sangon Biotech). The dissolved samples were used in western blotting. Protein concentration was determined using a BCA protein assay kit (Abcam, China). The proteins were transferred to nitrocellulose membranes (Bio-Rad, USA), and the membranes were blocked with 3% (w/v) milk (BD, USA) solution before incubation with monoclonal HRP-conjugated anti-6× His antibodies (Abcam, UK) diluted in 5% (w/v) BSA (Sangon Biotech). Protein signals were detected by HRP Substrate (Bio-Rad, USA) and Enhanced ECL Chemiluminescent Substrate (MKBio, China).
Propidium iodide (PI) assay
The PI assay was performed as described previously with small modifications [58]. Briefly, C. sakazakii were grown in LB broth up to the mid logarithmic phase, harvested, washed, and adjusted to 106 CFU/ml in LB at pH 3.0 or in LB at pH 7.0. The cells were incubated at 37°C for 2 hours. Subsequently, the cells were washed in PBS buffer and incubated with PI (1.3 μg/ml) at 37°C for 20 min in dark. A total of 10,000 cells were acquired for each flow cytometry analysis using a flow cytometer (Becton Dickinson).
Ethics statement
The study was approved by the Ethics Committee of our department, and written informed consent was obtained from all participants before the study.
Statistical analysis
Statistical analysis of all data was conducted using the GraphPad Prism program GraphPad (version 8.3). Significant differences were identified using Student’s two-tailed unpaired t-test (*, P < 0.05; **, P < 0.01; ***, P < 0.001, ns, not significant).
Supplementary Material
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2018YFC1604203) and the Fundamental Research Funds for the Central Universities, Nankai University (No. 63201218).
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 31772095)
Disclosure statement
The authors have no competing interests to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Elkhawaga AA, Hetta HF, Osman NS, et al. Emergence of Cronobacter sakazakii in cases of neonatal sepsis in Upper Egypt first report in North Africa. BMC Microbiol. 2020;11:215. .Epub 2019/12/29. 10.3389/fmicb.2020.00215. PubMed PMID: 32210926; PubMed Central PMCID: PMCPmc6935241 Pmc7075355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guo D, Wang S, Li J, et al. The antimicrobial activity of coenzyme Q(0) against planktonic and biofilm forms of Cronobacter sakazakii. Food Microbiol. 2020;86:103337. .Epub 2019/11/11. PubMed PMID: 31703870. [DOI] [PubMed] [Google Scholar]
- [3].Chen Z, Zhang Y, Lin R, et al. Cronobacter sakazakii induces necrotizing enterocolitis by regulating NLRP3 inflammasome expression via TLR4. J Med Microbiol. 2020;69(5):748–758. .Epub 2020/03/27. PubMed PMID: 32209170. [DOI] [PubMed] [Google Scholar]
- [4].Control CfD, Prevention . Enterobacter sakazakii infections associated with the use of powdered infant formula–tennessee, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(14):297. [PubMed] [Google Scholar]
- [5].Wang M, Wang L, Wu P, et al. Genomics and experimental analysis reveal a novel factor contributing to the virulence of cronobacter sakazakii strains associated with neonate infection. J Infect Dis. 2019;220(2):306–315. .Epub 2019/03/06. PubMed PMID: 30835279. [DOI] [PubMed] [Google Scholar]
- [6].Kim S, Yoon H, Ryu S.. New virulence factor CSK29544_02616 as lpxA binding partner in Cronobacter sakazakii. Sci Rep. 2018;8(1):835. .PubMed PMID: 29339761; PubMed Central PMCID: PMC5770445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jang H, Gopinath GR, Eshwar A, et al. The secretion of toxins and other exoproteins of Cronobacter: Role in virulence, adaption, and persistence. Microorganisms. 2020;8(2). 10.3390/microorganisms8020229. Epub 2020/02/13. PubMed PMID: 32046365; PubMed Central PMCID: PMCPmc7074816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Henry M, Fouladkhah A. Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms. 2019;7(3). 10.3390/microorganisms7030077.Epub 2019/03/16. PubMed PMID: 30870985; PubMed Central PMCID: PMCPmc6463179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Singh N, Goel G, Raghav M. Insights into virulence factors determining the pathogenicity of Cronobacter sakazakii. Virulence. 2015;6(5):433–440. .PubMed PMID: 25950947; PubMed Central PMCID: PMC4601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eshwar AK, Tasara T, Stephan R, et al. Influence of fkpA variants on survival and replication of Cronobacter spp. in human macrophages. Res Microbiol. 2015;166(3):186–195. .PubMed PMID: 25724920. [DOI] [PubMed] [Google Scholar]
- [11].Alvarez-Ordonez A, Cummins C, Deasy T, et al. Acid stress management by Cronobacter sakazakii. Int J Food Microbiol. 2014;178:21–28. .PubMed PMID: 24667315. [DOI] [PubMed] [Google Scholar]
- [12].Grant L, Cochran D. Can pH monitoring reliably detect gastro-oesophageal reflux in preterm infants? Arch Dis Childhood-Fetal Neonatal Ed. 2001;85(3):F155–F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhitnitsky D, Rose J, Lewinson O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci Rep. 2017;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim SA, Lee YM, Oh SW, et al. Biofilm formation and low pH viability of Cronobacter spp. (Enterobacter sakazakii) isolated from powdered infant formula and infant foods in Korea. Korean J Food Sci Anim Resour. 2009;29(6):702–708. [Google Scholar]
- [15].Tong L, Zhang M, Zhang X, et al. Exploration of factors in response to low acid tolerance using random mutagenesis in Cronobacter malonaticus. Food Res Int. 2019;116:994–999. [DOI] [PubMed] [Google Scholar]
- [16].Toyooka K, Takai S, Kirikae T. Rhodococcus equi can survive a phagolysosomal environment in macrophages by suppressing acidification of the phagolysosome. J Med Microbiol. 2005;54(11):1007–1015. [DOI] [PubMed] [Google Scholar]
- [17].Di A, Brown ME, Deriy LV, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8(9):933–944. [DOI] [PubMed] [Google Scholar]
- [18].Almajed FS, Forsythe SJ. Cronobacter sakazakii clinical isolates overcome host barriers and evade the immune response. Microb Pathog. 2016;90:55–63. .Epub 2015/12/01. PubMed PMID: 26616163. [DOI] [PubMed] [Google Scholar]
- [19].Ryan D, Ojha UK, Jaiswal S, et al. The small RNA dsrA influences the acid tolerance response and virulence of Salmonella enterica serovar Typhimurium. Front Microbiol. 2016;7:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang H, Song X, Wang P, et al. Expressed in response to the acidic pH in macrophages, promotes intracellular replication and virulence of Salmonella typhimurium. Int J Mol Sci. 2019;20(18):4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pereira SA, Â A, Ferreira V, et al. The impact of environmental stresses in the virulence traits of Listeria monocytogenes relevant to food safety. Listeria Monocytogenes. IntechOpen 2018;7:90–108. [Google Scholar]
- [22].Ji X, Lu P, van der Veen S. Development of a dual-antimicrobial counterselection method for markerless genetic engineering of bacterial genomes. Appl Microbiol Biotechnol. 2019;103(3):1465–1474. [DOI] [PubMed] [Google Scholar]
- [23].Hsieh CY, Wang JF, Huang PC, et al. Ralstonia solanacearum nlpD (rSc1206) contributes to host adaptation. Eur J Plant Pathol. 2012;133(3):645–656. [Google Scholar]
- [24].Du XJ, Fei W, Lu X, et al. Biochemical and genetic characteristics of Cronobacter sakazakii biofilm formation. Res Microbiol. 2012;163(6–7):448–456. [DOI] [PubMed] [Google Scholar]
- [25].Emami CN, Mittal R, Wang L, et al. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J Surg Res. 2012;172(1):18–28. .PubMed PMID: 21601887; PubMed Central PMCID: PMC3169739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kenney LJ. The role of acid stress in Salmonella pathogenesis. Curr Opin Microbiol. 2019;47:45–51. [DOI] [PubMed] [Google Scholar]
- [27].Chakraborty S, Winardhi RS, Morgan LK, et al. Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat Commun. 2017;8(1):1587. .PubMed PMID: 29138484; PubMed Central PMCID: PMC5686162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guzmán-Verri C, Manterola L, Sola-Landa A, et al. The two-component system Bvrr/BvrS essential for brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Nat Acad Sci. 2002;99(19):12375–12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hu WS, Li P-C, Cheng C-Y. Correlation between ceftriaxone resistance of Salmonella enterica serovar Typhimurium and expression of outer membrane proteins OmpW and Ail/OmpX-like protein, which are regulated by BaeR of a two-component system. Antimicrob Agents Chemother. 2005;49(9):3955–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu WS, Chen H-W, Zhang R-Y, et al. The expression levels of outer membrane proteins STM1530 and OmpD, which are influenced by the CpxAR and BaeSR two-component systems, play important roles in the ceftriaxone resistance of Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother. 2011;55(8):3829–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Naorem SS, Han J, Zhang SY, et al. Efficient transposon mutagenesis mediated by an IPTG-controlled conditional suicide plasmid. BMC Microbiol. 2018;18(1):158. .PubMed PMID: 30355324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Waterman SR, Small P. Acid-sensitive enteric pathogens are protected from killing under extremely acidic conditions of pH 2.5 when they are inoculated onto certain solid food sources. Appl Environ Microbiol. 1998;64(10):3882–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abeysundara PDA, Dhowlaghar N, Nannapaneni R. Influence of cold stress on the survival of listeria monocytogenes Bug600 and ScottA in lethal alkali, acid and oxidative stress. LWT. 2019;100:40–47. [Google Scholar]
- [34].Zhou A, Cao Y, Zhou D, et al. Global transcriptomic analysis of Cronobacter sakazakii CICC 21544 by RNA-seq under inorganic acid and organic acid stresses. Food Res Int (Ottawa, Ont). 2020;130:108963. .Epub 2020/03/12. PubMed PMID:32156398. [DOI] [PubMed] [Google Scholar]
- [35].Wang H, Wang X, Yu L, et al. Resistance of biofilm formation and formed-biofilm of Escherichia coli O157: H7 exposed to acid stress. LWT. 2020;118:108787. [Google Scholar]
- [36].de Freitas LL, Dos Santos CIA, Carneiro DG, et al. Nisin and acid resistance in Salmonella is enhanced by N-dodecanoyl-homoserine lactone. Microb Pathog. 2020:104320. 10.1016/j.micpath.2020.104320. [DOI] [PubMed] [Google Scholar]
- [37].Alvarez-Ordóñez A, Cummins C, Deasy T, et al. Acid stress management by Cronobacter sakazakii. Int J Food Microbiol. 2014;178:21–28. [DOI] [PubMed] [Google Scholar]
- [38].Zhang H, Hou A, Xie K, et al. Smart color-changing paper packaging sensors with pH sensitive chromophores based on azo-anthraquinone reactive dyes. Sens Actuators B Chem. 2019;286:362–369. [Google Scholar]
- [39].Tan H-Y, Wang N, Li S, et al. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Takaya A, Tomoyasu T, Matsui H, et al. The Dnak/ DnaJ chaperone machinery of Salmonella enterica serovar Typhimurium is essential for invasion of epithelial cells and survival within macrophages, leading to systemic infection. Infect Immun. 2004;72(3):1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Martina P, Milena G, Scinicariello S, et al. Author correction: The MFS efflux pump EmrKY contributes to the survival of shigella within macrophages. Sci Rep (Nature Publisher Group). 2019;9(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leuzzi R, Serino L, Scarselli M, et al. Ng‐MIP, a surface‐exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl‐prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol. 2005;58(3):669–681. [DOI] [PubMed] [Google Scholar]
- [43].Cianciotto NP, Fields BS. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Nat Acad Sci. 1992;89(11):5188–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tidhar A, Levy Y, Zauberman A, et al. Disruption of the NlpD lipoprotein of the plague pathogen Yersinia pestis affects iron acquisition and the activity of the twin-arginine translocation system. PLoS Negl Trop Dis. 2019;13(6):e0007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tsang M-J, Yakhnina AA, Bernhardt TG. NlpD links cell wall remodeling and outer membrane invagination during cytokinesis in Escherichia coli. PLoS Genet. 2017;13(7):e1006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rocaboy M. Structural and biochemical study of the proteins AmiC, NlpD and FtsW involved in the bacterial cell division: université de Liège. Liège, Belgique. Open Repository and Bibliography. 2013. [Google Scholar]
- [47].Yang LC, Gan YL, Yang LY, et al. Peptidoglycan hydrolysis mediated by the amidase AmiC and its LytM activator NlpD is critical for cell separation and virulence in the phytopathogen Xanthomonas campestris. Mol Plant Pathol. 2018;19(7):1705–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tidhar A, Flashner Y, Cohen S, et al. The Nlpd lipoprotein is a novel Yersinia pestis virulence factor essential for the development of plague. PloS One. 2009;4(9):e7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cumley NJ, Smith LM, Anthony M, et al. The Covs/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infect Immun. 2012;80(5):1650–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem. 2002;277(27):24155–24161. [DOI] [PubMed] [Google Scholar]
- [51].Samanta P, Clark ER, Knutson K, et al. OmpR and RcsB abolish temporal and spatial changes in expression of flhD in Escherichia coli biofilm. BMC Microbiol. 2013;13(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Prüß BM, Besemann C, Denton A, et al. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J Bacteriol. 2006;188(11):3731–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Walthers D, Go A, Kenney LJ. Regulation of porin gene expression by the two-component regulatory system EnvZ/OmpR. Bacterial and Eukaryotic Porins Structure, Function, Mechanism Wiley-VCH, Germany. 2004:1-24. [Google Scholar]
- [54].Li W, Ancona V, Zhao Y. Co-regulation of polysaccharide production, motility, and expression of type III secretion genes by EnvZ/OmpR and GrrS/GrrA systems in Erwinia amylovora. Mol Genet Genomics. 2014;289(1):63–75. [DOI] [PubMed] [Google Scholar]
- [55].Brzostek K, Skorek K, Raczkowska A. OmpR, a central integrator of several cellular responses in Yersinia enterocolitica. Adv Yersinia Res. 2012;954:325–334. [DOI] [PubMed] [Google Scholar]
- [56].Nadège P, Alcaraz JP, Coursange E, et al. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid. 2004;51(3):246–255. [DOI] [PubMed] [Google Scholar]
- [57].Qiu Y, Zhou YY, Kan B, et al. Construction of hemolysin hlyA gene knocked out of cholera vaccine candidate and inserted green fluorescent protein gene. J Pract Med. 2016;32(3):362–366. [Google Scholar]
- [58].Alvarez-Ordonez A, Begley M, Clifford T, et al. Transposon mutagenesis reveals genes involved in osmotic stress and drying in Cronobacter sakazakii. Food Res Int. 2014;55(jan):45–54. [Google Scholar]
- [59].Armbruster CE, Forsyth-DeOrnellas V, Johnson AO. Genome-wide transposon mutagenesis of Proteus mirabilis: Essential genes, fitness factors for catheter-associated urinary tract infection, and the impact of polymicrobial infection on fitness requirements. PLoS pathogens. 2017;13(6):e1006434. . PubMed PMID:28614382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bai Y, Yu H, Guo D, et al. Survival and environmental stress resistance of Cronobacter sakazakii exposed to vacuum or air packaging and stored at different temperatures. Front Microbiol. 2019;10:303. .Epub 2016/10/21. 10.3389/fmicb.2019.00303. PubMed PMID: 30842765; PubMed Central PMCID: PMCPmc6391331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vandal OH, Pierini LM, Schnappinger D, et al. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14(8):849–854. .PubMed PMID: 18641659; PubMed Central PMCID: PMC2538620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sivamaruthi BS, Madhumita R, Balamurugan K, et al. Cronobacter sakazakii infection alters serotonin transporter and improved fear memory retention in the rat. Front Pharmacol. 2015;6:188. .Epub 2015/09/22. PubMed PMID:26388777; PubMed Central PMCID:PMCPmc4560023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ripollés D, Harouna S, Parrón JA, et al. Inhibition of Cronobacter sakazakii adhesion to Caco-2 cells by commercial dairy powders and raw buttermilk. Journal of agricultural and food chemistry. 2017;65(5):1043–1050. . PubMed PMID:28092156. [DOI] [PubMed] [Google Scholar]
- [64].Gao JX, Li P, Du XJ, et al. A negative regulator of cellulose biosynthesis, bcsR, affects biofilm formation, and adhesion/invasion ability of Cronobacter sakazakii. Front Microbiol. 2017;8:1839. .Epub 2017/11/01. PubMed PMID: 29085341; PubMed Central PMCID:PMCPmc5649176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].de Lorenzo V, Eltis L, Kessler B, et al. Analysis of pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123(1):17–24. [DOI] [PubMed] [Google Scholar]
- [66].Huang Y-P, Li P, Du T, et al. Protective effect and mechanism of monascus-fermented red yeast rice against colitis caused by Salmonella enterica serotype Typhimurium ATCC 14028. Food Funct. 2020;11(7):6363–6375. [DOI] [PubMed] [Google Scholar]
- [67].van der Veen S, Abee T. Dependence of continuous-flow biofilm formation by Listeria monocytogenes EGD-e on SOS response factor YneA. Appl Environ Microbiol. 2010;76(6):1992–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59(12):4310–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


