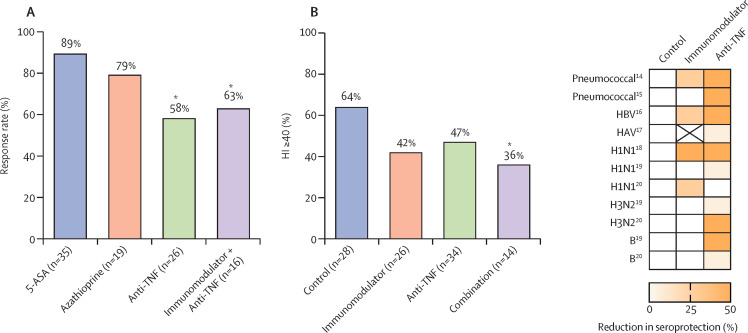
Figure 2.
Summary of studies of immunogenicity of vaccines in patients taking immunosuppressive therapies
(A) Pneumococcal vaccine response rate (response measured as a two-fold increase in anti-pneumococcal antibody titre) is reduced in patients administered anti-TNF monotherapy (58%) and immunomodulator and anti-TNF combination therapy (63%) relative to 5-ASA treated controls (89%).14 Asterisks denote statistically significant difference vs controls. (B) 2009 H1N1 influenza vaccination response rate (response measured as a ≥40% haemagluttinin inhibition [HI] titre) is attenuated in patients on immunomodulator and anti-TNF combination therapy (36%) relative to non-immunosuppressed controls (64%).18 Asterisk denotes statistically significant difference vs controls. (C) Heatmap adapted from studies of responses to vaccination in patients on immunomodulator or anti-TNF therapy showing percentage reduction in seroprotection to pneumococcal,14, 15 hepatitis B virus (HBV),16 hepatitis A virus (HAV),17 and influenza H1N1, H3N2, and B.19, 20 5=ASA=5-aminosalicylic acid.