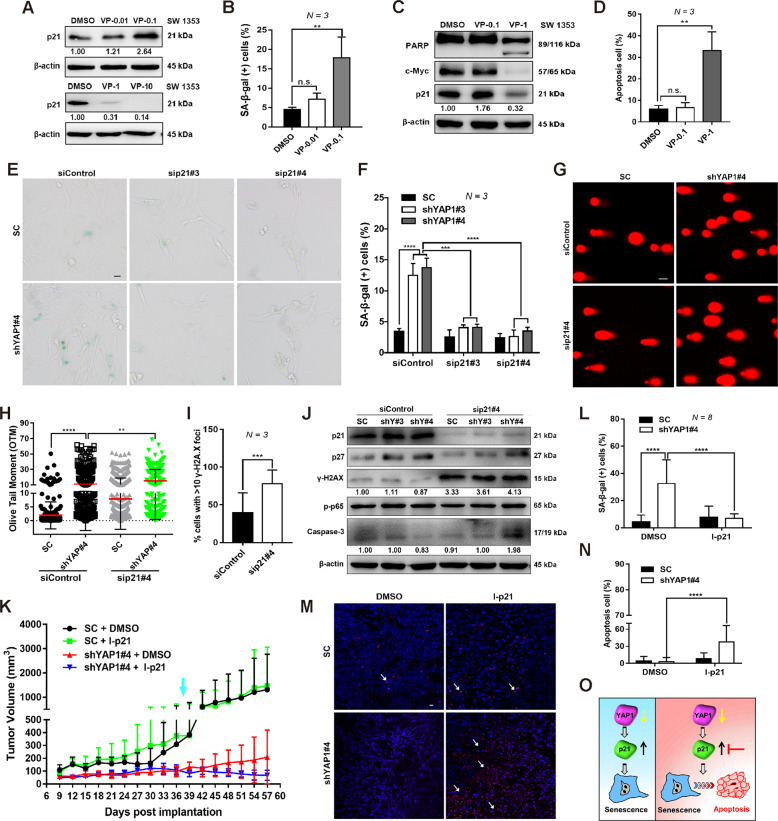
Fig. 4. p21 expression maintains the viability of YAP1 depletion-induced senescent cells.
A IB analysis of p21 expression in SW 1353 cells treated with DMSO or different concentrations of Verteporfin (0.01, 0.1, 1, and 10 μM) for 24 h. B The percentage of SA-β-gal-positive cells in the above treatments was quantified. C IB analysis of p21, c-Myc, and PARP expression in SW 1353 cells treated with DMSO or different concentrations of Verteporfin (0.1, 1 μM) for 24 h. D Flow cytometry analysis of cell apoptosis in the presence or absence of Verteporfin treatments. E, F Knockdown of p21 abolishes YAP depletion-induced senescence. Control shRNA or YAP1-depleted SW 1353 cells were transfected with p21-specific siRNAs (siControl, sip21#3 and sip21#4) for 72 h followed by SA-β-gal staining (E), and SA-β-gal-positive cells were quantified (F), scale bars: 20 μm. G, H p21 knockdown enhances YAP depletion-induced DNA damage. SW 1353 cells stably expressing control shRNA or shYAP1#4 were transfected with p21-specific siRNAs (siControl or sip21#4), and DNA damage was detected by a comet assay (g) and analyzed by the OTM (H), scale bars: 20 μm. I γ-H2AX foci were calculated in shYAP1#4 cells with/without p21 depletion. J Knockdown of p21 increases YAP1-depleted cell apoptosis. The expression of the indicated proteins was determined by IB. K SC and shYAP1#4 stable SW 1353 cells were subcutaneously inoculating into the nude mice at the right flank next to the forelimb, then the mice 37 days post inoculation (defined with light blue arrow) were treated with p21 inhibitor (I-p21, UC2288) for four times within 7 days, then tumor growth curves were constructed based on the tumor volumes measured every 3 days. L Frozen sections of fresh xenograft tumor were stained with SA-β-gal and the percentage of SA-β-gal-positive cells was determined. M, N Tumor sections of were stained with TUNEL (m, DAPI for nuclear staining) and the TUNEL-stained cells were quantified (N). O An illustration reveals that targeting p21 in YAP1-depleted cells switches cellular senescence to apoptosis. Data are presented as the mean ± SD of at least three independent experiments in (B, D, F, H, I, K, L, N). One-way ANOVA followed by Dunnett’s test was applied for (B, D, I). Student’s t-test for (I). Two-way ANOVA followed by Tukey’s test for (F, K, L, N). Mann–Whitney U test for (H). n.s. nonsignificant, **P < 0.01, ***P < 0.001, ****P < 0.0001.