Figure 1.
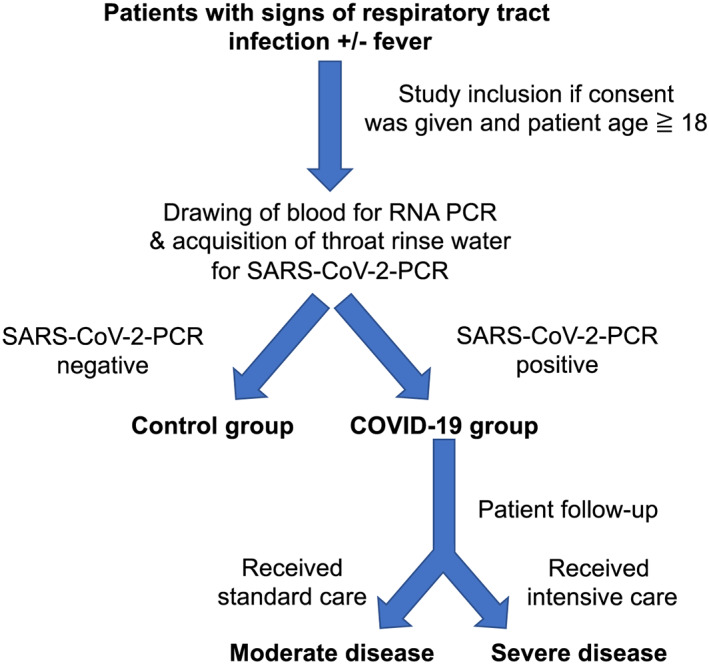
Overview of trial design. Patients were included in the study if they presented with signs of acute respiratory infection to the emergency department. Pre‐specified inclusion criteria were age ≥ 18 years and signs of acute respiratory infection. We included all patients with suspected COVID‐19 (defined by respiratory tract infection ± reported fever). For quantitative PCR analysis, patient blood was drawn by venepuncture from each consenting patient immediately after admission to the emergency department. Patients were then tested for SARS‐CoV‐2 infection. The patients with negative SARS‐CoV‐2 test result, who presented themselves with viral or bacterial respiratory tract infection, were used as control group. After consent, clinical baseline characteristics and vital signs were documented, and outcome and complications during hospital stay were assessed. Severe disease was defined as subsequent need for mechanical ventilation, admission to an intensive care unit, or death. Otherwise, (standard care) patients were classified as moderate disease.
