Abstract
The aim of this study was to assess the prognostic value of the steroid hormone receptor expression, counting the retinoid X receptor (RXR) and thyroid hormone receptors (THRs), on the two different breast cancer (BC) entities: multifocal/multicentric versus unifocal. The overall and disease-free survival were considered as the prognosis determining aspects and analyzed by uni- and multi-variate analysis. Furthermore, histopathological grading and TNM staging (T = tumor size, N = lymph node involvement, M = distant metastasis) were examined in relation to RXR and THRs expression. A retrospective statistical analysis was carried out on survival-related events in a series of 319 sporadic BC patients treated at the Department of Gynecology and Obstetrics at the Ludwig-Maximillian’s University in Munich between 2000 and 2002. The expression of RXR and THRs, including its two major isoforms THRα1 and THRα2, was analyzed by immunohistochemistry and showed to have a significant correlation for both BC entities in regard to survival analysis. Patients with multifocal/multicentric BC were exposed to a significantly worse disease-free survival (DFS) when expressing RXR. Patients with unifocal BC showed a significantly worse DFS when expressing THRα1. In contrast, a statistically significant positive association between THRα2 expression and enhanced DFS in multifocal/multicentric BC was shown. Especially the RXR expression in multifocal/multicentric BC was found to play a remarkably contradictory role for BC prognosis. The findings imply the need for a critical review of possible molecular therapies targeting steroid hormone receptors in BC treatment. Our results strengthen the need to further investigate the behavior of the nuclear receptor family, especially in relation to BC focality.
Keywords: breast cancer, focality, retinoid X receptor, thyroid hormone receptor, steroid hormone receptor, prognosis, unifocal, multifocal, multicentric
1. Introduction
Breast cancer (BC) is the most frequent malignant tumor in women worldwide [1]. With 2.1 million incident cases in 2018 [2,3], half a million deaths, and 14.9 million disability-adjusted life-years [4], breast cancer is considered one of the greatest challenges for experts to control [5]. Options for treatment of breast cancer have advanced greatly over the past years. The therapy intention is either of a curative nature or for the purpose of survival prolongation in metastatic BC and thus for the preservation of quality of life. Therapy regimes in adjuvant, neoadjuvant, and metastatic settings are immensely reliant on clinical tumor subtypes and include chemotherapy, surgery, aromatase inhibitors and hormone-receptor modulators [6,7,8].
For distinguishing clinical tumor subtypes, the BC focality is recognized as an important prognostic factor. The definition of multifocal BC states two or more separate tumor loci in the same quadrant. Multicentric BC is understood as two or more separate invasive tumors in more than one quadrant of the same breast [9,10]. To this point, no standard international definition has been implemented for distinguishing multifocal and multicentric BC [11,12]. Consequently, in our study, the multifocal and multicentric BC patients have been merged into one multifocal group to allow a distinct comparison with the unifocal BC patient group. The focality is a significant factor affecting the progressive course of disease and has been described by multiple studies for multifocal and multicentric BC patients [13]. Multifocality and/or multicentricity was found to be predictive of a worse prognosis through increased rates of distant metastasis, local relapse and shorter survival [14], prevalence of lymph node metastases [15], and higher mortality rates [16]. In contrast, unifocal BC is associated with an enhanced prognosis, including a better overall survival (OS) and disease-free survival (DFS), in comparison to multifocal and/or multicentric BC with the same tumor size. The focality is consequently regarded as an important prognosticator for BC [14,15,16].
The estrogen receptor (ER) and progesterone receptor (PR) are established “classical steroid hormone receptors” and are a key decision component in therapeutic approaches and forecasting prognosis for BC patients. There is strong evidence that the involvement of other nuclear receptors, besides ER and PR, play a vital role in breast cancer biology, including development and progression [17]. Personalized treatment options nowadays already involve drugs that target nuclear receptors [18]. The retinoid X receptor (RXR), thyroid hormone receptors (THRs), and Vitamin D receptor (VDR), are all members of the nuclear hormone receptor superfamily [19] and are ligand-dependent transcription factors, which bind several lipophilic hormones and lipid metabolites [20]. Our previous study has already identified the VDR in multifocal BC as an independent prognostic marker for a worsened OS. Interestingly, for the unifocal breast tumor patients, the VDR showed a significant positive association regarding the course of disease [21]. THRs have been identified to assemble with VDR and RXR by forming functional heterodimers. However, so far neither RXR nor THRs have been studied in association to breast cancer focality.
RXR and THRs activation is achieved by binding with its ligands and has been identified to form homodimers and heterodimers with many other members of the nuclear receptor superfamily [22]. After forming heterodimers [23], they (a) translocate to the nucleus, (b) bind to specific response elements upon promoters of specific genes, and (c) act as transcription factors [24]. Diverse ligands bound to these receptors, on the other hand, recruit different co-activators and consequently regulate different genes and biological functions [24].
Retinoids derived from vitamin A are signaling molecules that act via RXRs and are key components in cell differentiation and proliferation [25]. Retinoids have been previously described for their ability to induce differentiation and arrest proliferation in cancer cells [20,25]. To this point, three subtypes of the RXR have been identified: RXRα (NR2B1), RXRβ (NR2B2), and RXRγ (NR2B3) [26]. RXR is described to be expressed by breast cancer cells [27]. Increased expression of RXRα was identified in breast cancer cells rather than benign breast tissue [28]. These receptors are documented to have tumor suppressor properties in mediating the anti-proliferative effects of retinoic acid and inhibiting cell proliferation [25]. Evidence states that activation of RXR induces apoptosis in breast cancer cells and may reduce cell growth [29] in vitro and in animal models [30,31], also in combination with selective ER modulators [32,33]. Several studies, including Heublein et al., suggest that RXR positivity may predict favorable prognosis in breast cancer and comprise anti-cancer cell activity [34,35,36]. Overall, these findings suggest that RXR plays a key function in tumor pathogenesis.
Evidence implies a correlation between BC and thyroid disorders. Patients with thyroid dysfunctions show increased breast cancer incidences in contrast to healthy women [37,38]. Ditsch et al. [39] observed increased blood levels of the thyroid hormones (TH) fT3 and fT4 and concentrations of thyroid stimulating hormone (TSH) and antibodies against thyroidal peroxidase at the time of primary diagnosis in BC patients [40]. Circulating THs bind to THRs, endorse downstream signaling pathways and activate transcription factors [41]. Four major THRs isoforms have been identified: THRα1, THRα2, THRβ1, and THRβ2 [42]. THRα1, THRα2 and THRβ1 are overexpressed in several tissues in the human body except the liver as the major TH target organ [42,43]. Transcriptional activity is regulated by THRs through homo- or hetero-dimers with other nuclear receptors such as RXR and VDR. RXR forms a heterodimer with THRs and together influence downstream target gene expression by binding to specific DNA sequences that are located in regulatory regions identified as thyroid hormone elements [41,44,45]. THRs have been identified to be highly expressed in breast cancer tissue deriving from patients diagnosed with a BRCA1 germline mutation [34]. Further, THRs were of opposing prognostic significance and silencing of THRα appeared to diminish viability of BRCA1 mutated BC cells [46].
No study so far has identified the expression of RXR and THRs in human breast cancer specimens in regard to focality. New insights could potentially be promising in regard to cancer therapeutics. The above-mentioned nuclear receptors could consequently provide supplementary therapeutic targets for breast cancer patients.
Being aware of the immense development of todays clinical oncology towards customized treatment options, this study focused on investigating steroid hormone receptor expression in unifocal versus multifocal/multicentric sporadic BC and its influence on recurrence and survival. THRs and RXR, both nuclear receptors and activated by their steroid hormones, could be significant targets for generating new therapy treatments and prevention of BC. This study aims to provide a scientific base for future BC endocrine therapies adjusted to focality type, with the intetion to excert effectivness and decrease toxic treatment.
2. Results
2.1. Retinoid X Receptor (RXR)
2.1.1. Unifocal BC
Investigating the association of RXR expression on BC prognosis, no statistically significant difference was observed, neither for the OS (p = 0.360), nor for the DFS (p = 0.942), calculated by the log rank test. In addition, all three categories of TNM Staging (pT p = 0.440, pN p = 0.068, pM p = 0.673) and the histopathological grading by WHO (p = 0.738) revealed no significant difference between RXR positive or negative patients.
2.1.2. Multifocal and/or Multicentric BC
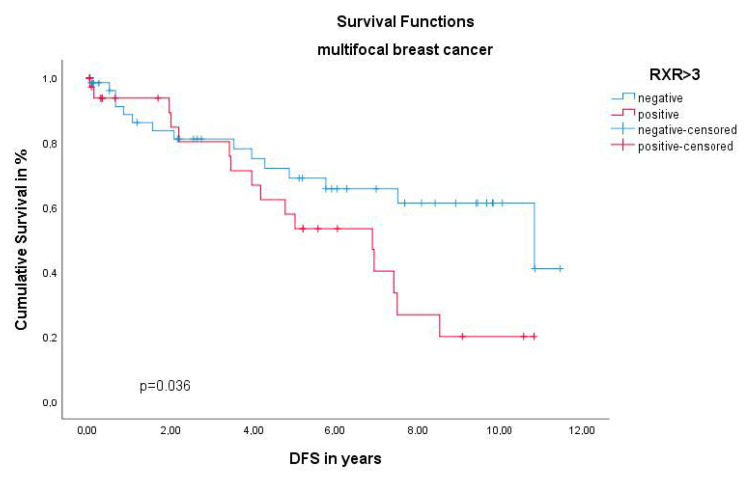
In multifocal and/or multicentric BC RXR expression showed no significant effect on the OS (p = 0.521). Yet, the Kaplan–Meier curve visualized (Figure 1) and the log rank test calculated with a p value of 0.036 show a significant negative association of the DFS in multifocal/multicentric BC patients when expressing the RXR. Interestingly the histopathological TNM staging (pT p = 0.328, pN = 0.820, pM = 0.497) and tumor grading by WHO (p = 0.466) Kruskal–Wallis analysis revealed no statistical difference for RXR expression in multifocal and/or multicentric BC. When conducting multivariate Cox regression, the RXR was identified as a dependent prognostic factor in the unifocal group for the DFS (HR 1.547, 95%CI 0.87–3.483, p = 0.292) (Table 1).
Figure 1.
Kaplan–Meier survival analysis among retinoid X receptor (RXR) positive and negative patients. Disease-free survival (DFS) of patients with multifocal and/or multicentric breast cancer (BC).
Table 1.
Multivariate Cox regression analysis of multifocal and/or multicentric BC patients regarding DFS.
| Variable. | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| Age | −0.001 | 0.999 (0.970–1.0301) | 0.967 |
| Grading | −0.008 | 0.992 (0.985–0.999) | 0.034 |
| pT | 0.215 | 1.240 (0.833–1.845) | 0.290 |
| pN | −0.003 | 0.997 (0.982–1.012) | 0.695 |
| pM | 2.353 | 10.516 (4.694–23.559) | 0.000 |
| RXR | 0.437 | 1.547 (0.687–3.483) | 0.292 |
Significant results are shown in bold; HR: hazard ratio; CI: confidence interval.
2.2. Thyroid Hormone Receptor α1 (THRα1)
2.2.1. Unifocal BC
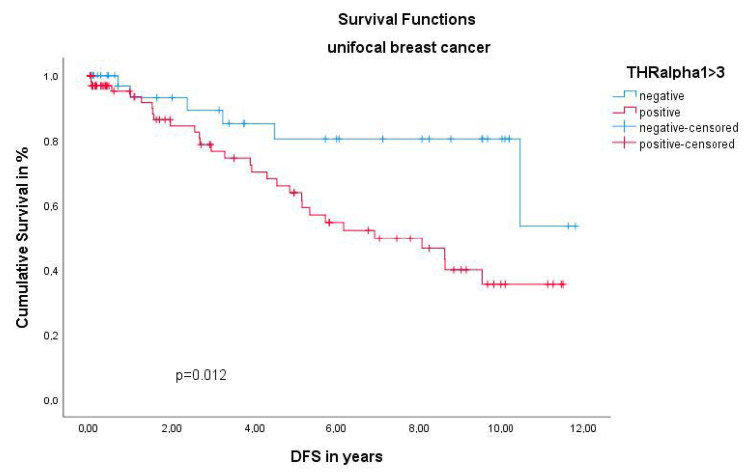
The THRα1 was the only receptor in this study, showing a significant effect on unifocal BC. The Kaplan–Meier curve illustrated a worse DFS for unifocal BC patients, when expressing the THRα1. This finding was confirmed by the Log-Rank test with a highly significant p value of 0.012 (Figure 2). Regarding the OS of unifocal BC patients, THRα1 expression revealed no statistically significant difference (p = 0.524). Additionally, no significant relation between THRα1 expression and TNM staging and WHO grading was calculated by Kruskal–Wallis analysis (pT p= 0.469; pN p = 0.464; pM p= 0.076; grading p = 0.470). Multivariate Cox regression did not identify the THRα1 as an independent prognostic factor for the DFS (HR 1.626, 95%CI 0.978–1.022, p = 0.721) (Table 2).
Figure 2.
Kaplan–Meier survival analysis among thyroid hormone receptor α1 (THRα1) positive and negative patients. DFS of patients with unifocal BC.
Table 2.
Multivariate Cox regression analysis of unifocal BC patients regarding DFS.
| Variable | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| Age | 0.010 | 1.010 (0.975–1.046) | 0.575 |
| Grading | 0.418 | 1.159 (0.761–3.033) | 0.236 |
| pT | 0.115 | 1.122 (0.784–1.605) | 0.530 |
| pN | 0.022 | 1.022 (0.795–1.314) | 0.867 |
| pM | 2.079 | 7.993 (3.007–21.248) | 0.000 |
| THRα1 | 0.486 | 1.626 (0.532–4.973) | 0.394 |
Significant results are shown in bold; HR: hazard ratio; CI: confidence interval.
2.2.2. Multifocal and/or Multicentric BC
Using the same statistical devices for the multifocal and/or multicentric BC group, no significant correlations between prognosis and THRα1 expression could be outlined (DFS p = 0.617; OS p = 0.564). Likewise, the Kruskal–Wallis tests revealed no significant results for tumor size pT (p = 0.479), involution of local lymph nodes (p = 0.255), the presence or lack of metastases (p = 0.494) or histopathological tumor grade (p = 0.325) at initial diagnosis for the multifocal and/or multicentric group.
2.3. Thyroid Hormone Receptor α2 (THRα2)
2.3.1. Unifocal BC
Unifocal BC patients revealed no significant correlations between THRα2 expression and prognosis in this study. Neither the OS (p = 0.199), nor the DFS (p = 0.243) were significantly affected by the THRα2, calculated by Log-Rank test. In line with these results, this receptor showed significant effects when tested for grading (p = 0.079) and staging (pT p = 0.699, pN p = 0.491, pM p = 0.180), calculated with the already mentioned statistical devices.
2.3.2. Multifocal and/or Multicentric BC
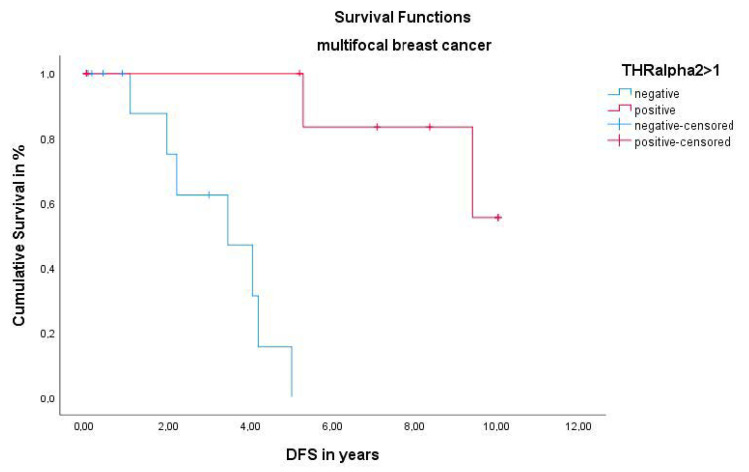
Like all the other analyzed receptors and cohorts, the OS was not affected by the lack or existence of the THRα2 (p = 0.053). A highly significant p value of 0.000 was calculated for the DFS by Log-Rank test. The Kaplan–Meier survival analysis visualized that patients with multifocal and/or multicentric BC have a better DFS, when expressing the THRα2 (Table 3). TNM Staging (pT p = 0.869, pN p = 0.069, pM p = 0.561) and WHO grading (p = 0.648) was, as well as all the receptors listed above, were not significant for the THRα2 in this cohort. In addition, the THRα2 was not an independent prognosticator for the DFS, when conducting multivariate COX regression (HR 0.742, 95% CI 0.370–1.486, p = 0.399) in the multifocal and/or multicentric group (Figure 3).
Table 3.
Multivariate Cox regression analysis of multifocal and/or multicentric BC patients regarding DFS.
| Variable | Coefficient | HR (95% CI) | p Value |
|---|---|---|---|
| Age | 0.005 | 1.005 (0.978–1.033) | 0.721 |
| Grading | −0.008 | 0.984 (0.984–0.999) | 0.033 |
| pT | 0.201 | 1.222 (0.841–1.776) | 0.293 |
| pN | −0.001 | 0.999 (0.984–1.014) | 0.880 |
| pM | 2.550 | 12.812 (5.662–28.988) | 0.000 |
| THRα2 | −0.299 | 0.742 (0.370–1.486) | 0.399 |
Significant results are shown in bold; HR: hazard ratio; CI: confidence interval.
Figure 3.
Kaplan–Meier survival analysis among THRα2 positive and negative patients. DFS of patients with multifocal and/or multicentric BC.
3. Discussion
The aim of this study was to evaluate the prognostic association of the steroid hormone receptor RXR and THRs expression in relation to the two different BC entities: unifocal vs. multifocal BC. Understanding the mechanisms by which RXRs and THRs exert their effects in breast cancer patients remains incomplete [25]. This is the first study to define the prognostic role of THRs and RXR in breast cancer in relation to the two different BC entities, using a relatively large clinical patient cohort with long-term follow up. Results from the current study provide evidence that expression of RXR and THRs showed a significant association in terms of the course of BC disease in relation to focality.
RXR and THRs, both nuclear receptors with their associated ligands, operate as potent regulators of cell differentiation, development, and normal physiology. Furthermore, they might play an important role in different pathologies including breast cancer [22,47]. As previously described, the RXR, THR, and VDR have been identified to form functional homodimers and heterodimers with many other members of the nuclear receptor superfamily, also in human breast cancer cell lines [22]. These were able to mediate selective responses such as growth inhibition and apoptosis, supporting initially a protective role for breast cancer development [30]. As these molecules are considered as potential targets for molecular therapy [29,30], the aim of these studies was to evaluate the RXR and TRHs expression in tissue of BC patients and to correlate it with major clinicopathological characteristics and prognostic factors, in relation to BC focality. Evaluation of the immunoreactive score of Remmele and Stegner (IRS) was performed as both RXR and TRH belong to the nuclear receptors family for which the IRS is commonly used [48].
The nuclear receptor RXR has been proven to modulate cellular differentiation and apoptosis in different tumor entities [49]. RXR and its heterodimers regulate the function of myeloid cells, link cellular metabolism, and show a profound effect on immune function [50,51]. Previous studies have concluded that the specific activation of RXR may up regulate chemokine expression and promote phagocytosis of apoptotic cells. Further, it can decrease antiviral responses in myeloid cells [50,52]. Increased expression of RXR has been related with an up-regulated apoptosis in ovarian cancer and a specific RXR activation in an ovarian tumor-model even showed an apoptosis re-activation [53]. In lung cancer, the epigenetic silencing of RXR was associated with decreased OS while the exact mechanism for this result is still unknown [54]. In in vitro studies on BC, RXR ligands or retinoids are reported to induce apoptosis in BCL2-positive human cancer cells [29], and decreased vascularization in BC tumors in transgenic mice [55]. Furthermore, RXR agonist therapy also suppressed mammary tumorigenesis in transgenic mice [56]. Additionally, RXR activation down regulated the COX-2 expression in BC cells [57], and blocked the BC cell cycle at the G1 phase [58]. Nevertheless, in a clinical trial for treating metastatic BC, a retinoid agonist was ineffective [59]. On the other hand, BRCA1 mutated breast cancer cells have been hypothesized to be sensitive to RXR and VDR modulating drugs [34].
Interestingly, the results of our study do not support the tumor-inhibiting role of RXR. Patients with multifocal/multicentric BC showed a significantly worse DFS when expressing RXR. In contrast, no significant correlation between RXR expression was noted in survival analysis for unifocal BC. In addition, no correlation between RXR expression and TNM staging or grading was found. In line with our previous findings, also the VDR expression showed to play a remarkably paradox role for BC prognosis. The multifocal/multicentric BC patients with significantly worse DFS revealed enhanced expression levels of the VDR. We even identified the VDR to be an independent prognostic marker for multifocal BC patients [21]. We propose that increased expression of RXR might potentiate heterodimer formation and activation of other nuclear receptors such as VDR, thus increasing a possible tumorigenic function in multifocal BC. We therefore suggest a fundamental interaction between RXR and VDR and their heterodimers in multifocal BC patients.
Despite previous histological data supporting an anti-tumorigenic effect of elevated RXR and VDR expression, the findings of our study group seem to rather support the opposite in multifocal BC. Since the exact mechanism is still unclear, these data strengthen the need to further investigate the behavior of the nuclear receptor family including RXR and VDR in BC, especially in relation to focality. Due to the application of the IRS, our findings should be considered with great care. If, however, this holds true if proven by larger series, we suggest a critical reconsideration of possible RXR and Vitamin D therapy approaches subjected to down-regulation along the BC progression and continue further research of the steroid hormonal receptor pathogenesis in BC subtypes.
Likewise, THRs form homodimers or heterodimers with RXR and are later activated by thyroid hormones. Consequently, they act as classical transcription factors by binding to the promoter regions of target genes [60]. THRs are encoded by two genes: THRα and THRβ—on chromosome 17 and 3, respectively [60,61,62]. To this date, there is still little knowledge regarding the specific THRα isoforms: THRα1 and THRα2. As described previously, THRα is expressed in various organ tissues and its analogous malignant tissues, yet its clinical relevance and role in BC etiology and progression remains unclear [63,64,65,66]. The functional similarity of THRs and ER/PR have previously led to the hypothesis that THRs may be a prognostic marker in breast cancer patients [67]. Ditsch et al. revealed an association between lower THRα2 expression and worse survival outcome in general BC patients [68]. Another study by Conde et al. exposed a significant correlation between high THRα expression and DFS in BC patients, however, without assessing the specific THRα isoforms individually [64].
In our study, THRα1 and THRα2 showed a prognostic association between both BC entities, but with major differences. Patients with unifocal BC showed a significantly worse DFS when expressing THRα1. In contrast, no significant correlation between THRα1 expression was noted in survival analysis for multifocal/multicentric BC. On the other hand, there was a statistically significant positive association between high THRα2 expression and enhanced DFS in multifocal/multicentric BC. No statistically significant association was found for unifocal BC and THRα2 expression. There was no significant correlation of the THRα isoforms concerning TNM, histopathological grading, and staging. Our findings are congruent with previous outcomes regarding THRα expression and their effect on survival analysis in BC. Similarly, Jerzak et al. described low THRα1 expression and high THRα2 expression in general BC patients that had the highest observed 5-year OS [63]. Nevertheless, our study, for the first time, identified THRα expression in human breast cancer specimens concerning focality.
Opposing molecular pathways of the THRα isoforms may explain the cause for differing effects on survival analysis. Whilst THRα1 is activated by thyroid hormone [69], THRα2 lacks the binding site for thyroid hormone [63,70]. In detail, THRα2 serves as an antagonist of thyroid hormone-mediated biological effects and signaling, preventing over- or under-activity of thyroid-resolved effects. Based on our results, expression of THRα2 may antagonize signaling of thyroid hormone growth-promoting effects that are mediated by THRα1 [42,70,71]. While the mechanism underlying this finding has not been determined, it is possible that unifocal BC patients with predominant THRα1 expression may benefit from reducing thyroid hormone concentrations and/or inhibiting THRα1 [72,73]. It is hypothesized that THRα2 expression reduces growth-promoting genes in breast cancer by decreased transcription of p53 and retinoblastoma [74]. We hypothesize that multifocal/multicentric BC patients may profit from the up-regulation of THRα2. Especially for these BC focality entities, with an unfavorable prognosis; the survival outcomes could be improved [75].
Distinguishing the BC entities may be regarded as the most important limitation of this study. Dividing BC entities into its subtypes may differ depending on pathological centers and examiners. Especially for multifocal BC, defined by two or more separate tumor loci in the same quadrant, minimal distance between the separate tumors may result to be considered as unifocal BC. Thus, distinction between unifocal and multifocal BC may not always be clear. Additionally, to date, no standard international definition has been implemented for distinguishing multifocal and multicentric BC [11,12]. So far, several studies including Weissenbacher et al. have hypothesized that the two entities were found to be predictive of a worse prognosis. The question of whether multifocal and multicentric BC can be regarded as equivalent in terms of aggressiveness of the disease should become the subject of further investigations.
4. Materials and Methods
4.1. Patients
The cohort for this study is built of patients with BC treated in the years of 2000 to 2002 at the Department of Gynecology and Obstetrics at the Ludwig-Maximillian’s University in Munich.
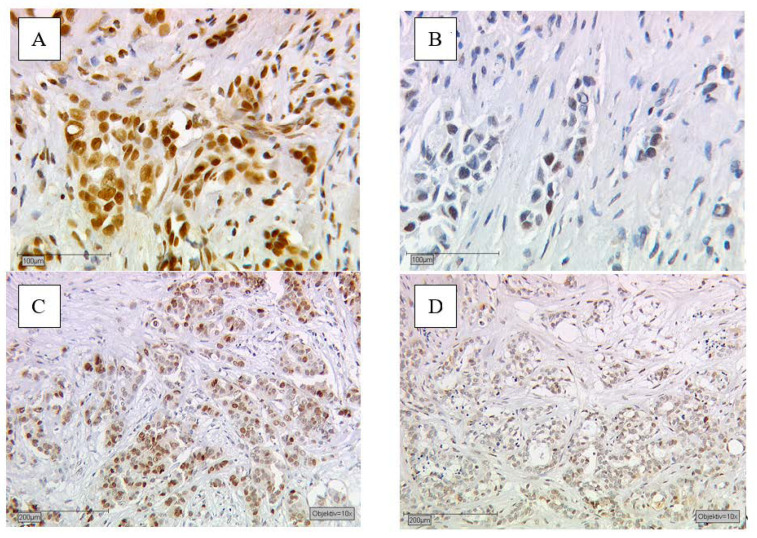
The study’s aim was to assess the prognostic value of steroid hormone receptors on the two different BC entities: unifocal vs. multifocal and/or multicentric sporadic BC. According to the current state of research, we decided to include and further investigate the most relevant steroid hormone receptors: RXR and THRs and here both alpha isoforms, counting THRα1 and THRα2. As described in Section 4.2, selected samples were immunohistochemically stained (Figure 4) and statistically analyzed in the manner described Section 4.3.
Figure 4.
Shows immunohistochemical staining of RXR and THRα2 after incubation with the primary antibody of the malignant breast cancer cells. Immunohistochemical staining of RXR in human BC (A,B). (A) With an immunoreactive score of Remmele and Stegner (IRS) of 8 meaning RXR positive and (B) an IRS value ≤ 2 being RXR negative. Immunohistochemical staining of THRα2 in BC (C,D). (C) With an IRS of 9 meaning THRα2 positive and (D) an IRS value ≤ 1 being THRα2 negative.
To determine the focality, the recruits had to undergo set clinical diagnostics: clinical examination, ultrasound and X-ray. If the identification of the focality still was indistinct, further diagnostics such as nuclear magnetic resonance imaging (NMRI), pneumocystography or galactography, were added. Finally, we excluded patients with an unclear receptor status and/or focality type from our database. In the end, 319 patients all meeting the set requirements built our total collective (TC) (Table 4). With the TC database, survival analysis was performed for each receptor and always in regard to the focality. After an observation period of up to 10 years DFS and OS were statistically analyzed, this follow-up data were retrieved from the Munich Cancer Registry.
Table 4.
Patient characteristics of the total collective.
| Patient Characteristics | n (%) |
|---|---|
| Age (years) | Median 59.09 Range 69 |
| Tumor foci | Unifocal 173 (54.2) Multifocal 146 (45.7) |
| Histology | NST 188 (61.4) Non-NST 118 (38.5) |
| Tumor grade | G1 or G2 165 (52.2) G3 151 (47.7) |
| pT | pT1 197 (64.3) pT2-pT4 109 (35.6) |
| pN | pN0 166 (54.2) pN1-pN3 140 (45.7) |
| pM | pM0 239 (78.1) pM1 67 (21.8) |
| RXR | negative 186 (58.3) positive 133 (41.6) |
| THRα1 | negative 120 (37.6) positive 199 (62.3) |
| THRα2 | negative 172 (53.9) positive 147 (46.0) |
For further investigation, e.g., for the TNM staging [76,77], WHO grading [78] and multivariate analysis, the TC was subdivided into two groups, depending on the focality. Group 1 contained all patients with unifocal BC with a total of 173 patients. To make it clearer and more comprehensible, we merged the multifocal and multicentric BC cases to Group 2, including 146 patients.
In Table 4, detailed patient characteristics from the TC are summarized and displayed. The large TC of 319 patients and the relatively equal distribution in the subgroups strengthen the statistical power of our study. The median age at initial diagnosis from the 319 patients included was 59 years with a range of 69. Overall, 173 patients were diagnosed with unifocal and 146 with multifocal and/or multicentric BC; 61.4% of the patients had histological invasive carcinoma of no special type (NST); 52.2% of our patients had a low-grade carcinoma (G1-G2 = 52.2%, G3 = 47.7%) and 64.3% were staged with a tumor size smaller than 2 cm (pT1: 64.3%, pT2-pT4: 35.6); 54.2% of all patients were staged pN0. The majority of our TC was staged with no present metastasis at initial diagnosis (pM0 = 78.1%, pM1 = 21.8%). The negligibly different total patient numbers (N) in the subgroups may be explained by the lack of a limited number of input variables that could not be obtained by the retrospective character of the study.
4.2. Immunohistochemistry
According to the earlier published and well described methods [46,68,79], immunohistochemistry of RXR and THRα on formalin-fixed, paraffin-embedded sections was performed. Therefore, a combination of pressure cooker heating and the standard streptavidin–biotin–peroxidase complex with mouse/rabbit-IgG-Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) were used. For the staining, we utlizied the following anibodies: the THRα1 was stained with polyclonal rabbit IgG antibodies (AbD Serotec Oxford, UK), the THRα2 by using monoclonal rabbit IgG1 (AbD Serotec, Oxford, UK) Abcam, Cambridge, UK); Zytomed, Berlin, Germany) and the RXR was detected by monoclonal mouse antibodies (Perseus Proteomics Inc., Tokyo, Japan). For the negative controls, instead of the primary antibody we used appropriate tissue sections, which were treated with pre-immune IgGs (supersensitive rabbit negative control, BioGenex, Fremont, CA, USA). Figure 4 contains an exemplary presentation of immunohistochemical stained steroid receptors. Positive-stained tissue appeared in a brownish color (Figure 4A,C) and negative as well as unstained cells appeared in blue (Figure 4B,D).
To quantify the immunoreactivity, meaning the distribution and intensity patterns, two blinded and independent observers evaluated via semi-quantitative immunoreactive score of Remmele and Stegner (IRS) [48] by using a Leitz microscope (Wetzlar, Germany) and a 3CCD color camera (JVC, Victor company of Japan, Yokohama, Japan). The IRS scoring system ranges from 0 to 12. Therefore, the staining intensity (Score 0 = no staining, Score 1 = weak staining, Score 2 = moderate staining, Score 3 = strong staining) needed to be multiplied with the percentage of positively stained cells.
Tissue samples that had been assigned an IRS greater than 3 for the RXR and THR α1 were scored as positive. For the THR α2 we assessed an IRS higher than 1 to be positive.
4.3. Statistical Analysis
In this study, statistical analysis was performed by using the IBM Statistical Package for the Social Sciences (IBM SPSS Statistic 24.0 Inc., Chicago, IL, USA). The collected results were inserted into the SPSS database in the implied manner, building the TC.
Using the TC database, we analyzed the effect of the initially defined three receptors on the OS and DFS always with regard to the focality. By applying the chi-square of the log rank test, we tested for significance. Kaplan–Meier survival analysis was performed for the visualization of each steroid receptor. For the statistical evaluation of the TNM staging and histopathological WHO grading, we divided the TC database into two subgroups due to the BC focality: Database 1 including all patients with unifocal BC and Database 2 containing all patients with multifocal and/or multicentric BC. Here, the nonparametric Kruskal–Wallis test for significance and boxplots were used to examine variables. Multivariate analyses via Cox regression evaluated the dependency as a prognostic marker of each receptor, when adjusted for age, staging and grading. Each parameter to be considered significant in our study was required to have a p value less than 0.05.
5. Conclusions
In conclusion, the present study analyzed the prognostic association of the steroid hormone receptors’ expression of RXR and THRα in relation to the different BC entities: unifocal vs. multifocal/multicentric BC. The RXR expression was shown to play a remarkably incongruous role for BC prognosis in comparison to previous findings. Patients with multifocal/multicentric BC were exposed to a significantly worse DFS when expressing RXR. In line with our previous findings, also the VDR expression previously showed to play a remarkably contradictory role for BC prognosis [21]. Despite previous reports supporting an anti-tumorigenic effect of elevated RXR and VDR expression, our results seem to rather support the opposite effect for multifocal BC patients. The findings imply a critical review of possible molecular therapies involving RXR and Vitamin D as a target in BC treatment. If proven by larger series, future therapy decisions should be made in hindsight of BC focality. THRα1 and THRα2 showed a prognostic association in both BC entities, but with major differences. Patients with unifocal BC showed a significantly worse DFS when expressing THRα1. In contrast, a statistically significant positive association between THRα2 expression and enhanced DFS in multifocal/multicentric BC was shown. Our findings are congruent with previous outcomes regarding THRα expression and its effect on survival analysis in breast cancer. Our study, for the first time, identified THRα expression in human breast cancer specimens in regard to focality. In summary, thyroid hormone-modulated therapies should become the subject of further investigations in regard to BC focality. Our results strengthen the need to further investigate the behavior of the nuclear receptor family in BC, especially in relation to focality. Further examinations studying the cause and to what extent BC focality may impact hormonal effects would be of major interest.
Abbreviations
| BC | Breast cancer |
| DFS | Disease-free survival |
| ER | Estrogen receptor |
| IRS | Immmunoreactive score |
| PR | Progesterone receptor |
| OS | Overall survival |
| RXR | Retinoid X receptor |
| TC | Total collective |
| TH | Thyroid hormone |
| THR | Thyroid hormone receptor |
| THRα | Thyroid hormone receptor alpha |
| THRα1 | Thyroid hormone receptor alpha 1 |
| THRα2 | Thyroid hormone receptor alpha 2 |
| VDR | Vitamin D receptor |
Author Contributions
U.J. and N.D. conceived and designed the project. A.Z.Z. and T.V. wrote the paper and carried out the statistical evaluation. T.V. and U.J. provided the concept, substantially contributed to manuscript and supervised the research. J.-N.M., S.-N.J., A.V., T.K., F.B., S.S. and H.H.H. revised the manuscript for critical content and helped with statistical evaluation. Funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by internal departmental sources.
Institutional Review Board Statement
The current study was approved by the Ethics Committee of the Ludwig-Maximilian-University Munich, Germany (approval number 048–08) on the 5th of February 2018.
Informed Consent Statement
All patients gave their consent to participate in the study.
Data Availability Statement
Data are accessible on request from the authors.
Conflicts of Interest
Sven Mahner: Research support, advisory board, honoraria and travel expenses from AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Medac, MSD, Novartis, Olympus, PharmaMar, Roche, Sensor Kinesis, Teva, Tesaro; All other authors declare no conflict of interest.
Ethics Approval and Consent to Participate
The tissue samples used in this study were left over material after all diagnostics had been completed and were retrieved from the archive of Gynecology and Obstetrics, Ludwig-Maximilian-University, Munich, Germany. All patients gave their consent to participate in the study. All patient data were fully anonymized, the study was performed according to the standards set in the declaration of Helsinki 1975. The current study was approved by the Ethics Committee of the Ludwig-Maximilian-University Munich, Germany (approval number 048–08, 05 February 2018). Authors were blinded from the clinical information during experimental analysis.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Cancer Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, 2015. Adv. Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B., Costantino J.P., Wickerham D.L., Cecchini R.S., Cronin W.M., Robidoux A., Bevers T.B., Kavanah M.T., Atkins J.N., Margolese R.G., et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 7.Cauley J.A., Norton L., Lippman M.E., Eckert S., Krueger K.A., Purdie D.W., Farrerons J., Karasik A., Mellstrom D., Ng K.W., et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res. Treat. 2001;65:125–134. doi: 10.1023/A:1006478317173. [DOI] [PubMed] [Google Scholar]
- 8.Goss P.E., Ingle J.N., Ales-Martinez J.E., Cheung A.M., Chlebowski R.T., Wactawski-Wende J., McTiernan A., Robbins J., Johnson K.C., Martin L.W., et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 9.Muller K., Sixou S., Kuhn C., Jalaguier S., Mayr D., Ditsch N., Weissenbacher T., Harbeck N., Mahner S., Cavailles V., et al. Prognostic relevance of RIP140 and ERbeta expression in unifocal versus multifocal breast cancers: A preliminary report. Int. J. Mol. Sci. 2019;20:418. doi: 10.3390/ijms20020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ataseven B., Lederer B., Blohmer J.U., Denkert C., Gerber B., Heil J., Kuhn T., Kummel S., Rezai M., Loibl S., et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6,134 breast cancer patients treated with neoadjuvant chemotherapy. Ann. Surg. Oncol. 2015;22:1118–1127. doi: 10.1245/s10434-014-4122-7. [DOI] [PubMed] [Google Scholar]
- 11.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF) Interdiszipliäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. [(accessed on 21 December 2020)]; Available online: https://www.awmf.org/uploads/tx_szleitlinien/032-045OLl_S3_Mammakarzinom_2017-12.pdf.
- 12.Shaikh T., Tam T.Y., Li T., Hayes S.B., Goldstein L., Bleicher R., Boraas M., Sigurdson E., Ryan P.D., Anderson P. Multifocal and multicentric breast cancer is associated with increased local recurrence regardless of surgery type. Breast J. 2015;21:121–126. doi: 10.1111/tbj.12366. [DOI] [PubMed] [Google Scholar]
- 13.Zati Zehni A., Jeschke U., Hester A., Kolben T., Ditsch N., Jacob S.N., Mumm J.N., Heidegger H.H., Mahner S., Vilsmaier T. EP3 Is an Independent Prognostic Marker Only for Unifocal Breast Cancer Cases. Int. J. Mol. Sci. 2020;21:4418. doi: 10.3390/ijms21124418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissenbacher T.M., Zschage M., Janni W., Jeschke U., Dimpfl T., Mayr D., Rack B., Schindlbeck C., Friese K., Dian D. Multicentric and multifocal versus unifocal breast cancer: Is the tumor-node-metastasis classification justified? Breast Cancer Res. Treat. 2010;122:27–34. doi: 10.1007/s10549-010-0917-9. [DOI] [PubMed] [Google Scholar]
- 15.Lang Z., Wu Y., Li C., Li X., Wang X., Qu G. Multifocal and Multicentric Breast Carcinoma: A Significantly More Aggressive Tumor than Unifocal Breast Cancer. Anticancer Res. 2017;37:4593–4598. doi: 10.21873/anticanres.11858. [DOI] [PubMed] [Google Scholar]
- 16.Boros M., Voidazan S., Moldovan C., Georgescu R., Toganel C., Moncea D., Molnar C.V., Podoleanu C., Eniu A., Stolnicu S. Clinical implications of multifocality as a prognostic factor in breast carcinoma—A multivariate analysis study comprising 460 cases. Int. J. Clin. Exp. Med. 2015;8:9839–9846. [PMC free article] [PubMed] [Google Scholar]
- 17.Hua S., Kittler R., White K.P. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscat G.E., Eriksson N.A., Byth K., Loi S., Graham D., Jindal S., Davis M.J., Clyne C., Funder J.W., Simpson E.R., et al. Research resource: Nuclear receptors as transcriptome: Discriminant and prognostic value in breast cancer. Mol. Endocrinol. 2013;27:350–365. doi: 10.1210/me.2012-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escriva H., Bertrand S., Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- 20.Dawson M.I., Xia Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta. 2012;1821:21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zati Zehni A., Jacob S.N., Mumm J.N., Heidegger H.H., Ditsch N., Mahner S., Jeschke U., Vilsmaier T. Hormone Receptor Expression in Multicentric/Multifocal versus Unifocal Breast Cancer: Especially the VDR Determines the Outcome Related to Focality. Int. J. Mol. Sci. 2019;20:5740. doi: 10.3390/ijms20225740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditsch N., Vrekoussis T., Lenhard M., Ruhl I., Gallwas J., Weissenbacher T., Friese K., Mayr D., Makrigiannakis A., Jeschke U. Retinoid X receptor alpha (RXRalpha) and peroxisome proliferator-activated receptor gamma (PPARgamma) expression in breast cancer: An immunohistochemical study. In Vivo. 2012;26:87–92. [PubMed] [Google Scholar]
- 23.Forman B.M., Umesono K., Chen J., Evans R.M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 24.Koeffler H.P. Peroxisome proliferator-activated receptor gamma and cancers. Clin. Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 25.Tang X.H., Gudas L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 26.Auwerx J., Baulieu E., Beato M., Becker-Andre M., Burbach P.H., Camerino G., Chambon P., Cooney A., Dejean A., Dreyer C., et al. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 27.Raffo P., Emionite L., Colucci L., Belmondo F., Moro M.G., Bollag W., Toma S. Retinoid receptors: Pathways of proliferation inhibition and apoptosis induction in breast cancer cell lines. Anticancer Res. 2000;20:1535–1543. [PubMed] [Google Scholar]
- 28.Friedrich M., Axt-Fliedner R., Villena-Heinsen C., Tilgen W., Schmidt W., Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor alpha in breast cancer. Histochem. J. 2002;34:35–40. doi: 10.1023/A:1021343825552. [DOI] [PubMed] [Google Scholar]
- 29.Elstner E., Williamson E.A., Zang C., Fritz J., Heber D., Fenner M., Possinger K., Koeffler H.P. Novel therapeutic approach: Ligands for PPARgamma and retinoid receptors induce apoptosis in bcl-2-positive human breast cancer cells. Breast Cancer Res. Treat. 2002;74:155–165. doi: 10.1023/A:1016114026769. [DOI] [PubMed] [Google Scholar]
- 30.Crowe D.L., Chandraratna R.A. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6:R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh N., Wang Y., Williams C.R., Risingsong R., Gilmer T., Willson T.M., Sporn M.B. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–5673. [PubMed] [Google Scholar]
- 32.Zanardi S., Serrano D., Argusti A., Barile M., Puntoni M., Decensi A. Clinical trials with retinoids for breast cancer chemoprevention. Endocr. Relat. Cancer. 2006;13:51–68. doi: 10.1677/erc.1.00938. [DOI] [PubMed] [Google Scholar]
- 33.Ross-Innes C.S., Stark R., Holmes K.A., Schmidt D., Spyrou C., Russell R., Massie C.E., Vowler S.L., Eldridge M., Carroll J.S. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24:171–182. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heublein S., Mayr D., Meindl A., Kircher A., Jeschke U., Ditsch N. Vitamin D receptor, Retinoid X receptor and peroxisome proliferator-activated receptor gamma are overexpressed in BRCA1 mutated breast cancer and predict prognosis. J. Exp. Clin. Cancer Res. 2017;36:57. doi: 10.1186/s13046-017-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonofiglio D., Cione E., Vizza D., Perri M., Pingitore A., Qi H., Catalano S., Rovito D., Genchi G., Ando S. Bid as a potential target of apoptotic effects exerted by low doses of PPARgamma and RXR ligands in breast cancer cells. Cell Cycle. 2011;10:2344–2354. doi: 10.4161/cc.10.14.15917. [DOI] [PubMed] [Google Scholar]
- 36.Yasmin R., Kannan-Thulasiraman P., Kagechika H., Dawson M.I., Noy N. Inhibition of mammary carcinoma cell growth by RXR is mediated by the receptor’s oligomeric switch. J. Mol. Biol. 2010;397:1121–1131. doi: 10.1016/j.jmb.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuijpens J.L., Nyklictek I., Louwman M.W., Weetman T.A., Pop V.J., Coebergh J.W. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid. 2005;15:1253–1259. doi: 10.1089/thy.2005.15.1253. [DOI] [PubMed] [Google Scholar]
- 38.Turken O., NarIn Y., DemIrbas S., Onde M.E., Sayan O., KandemIr E.G., Yaylac I.M., Ozturk A. Breast cancer in association with thyroid disorders. Breast Cancer Res. 2003;5:R110–R113. doi: 10.1186/bcr609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ditsch N., Liebhardt S., Von Koch F., Lenhard M., Vogeser M., Spitzweg C., Gallwas J., Toth B. Thyroid function in breast cancer patients. Anticancer Res. 2010;30:1713–1717. [PubMed] [Google Scholar]
- 40.Tremmel E., Hofmann S., Kuhn C., Heidegger H., Heublein S., Hermelink K., Wuerstlein R., Harbeck N., Mayr D., Mahner S., et al. Thyronamine regulation of TAAR1 expression in breast cancer cells and investigation of its influence on viability and migration. Breast Cancer. 2019;11:87–97. doi: 10.2147/BCTT.S178721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y.C., Yeh C.T., Lin K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019;20:4986. doi: 10.3390/ijms20204986. [DOI] [Google Scholar]
- 42.Mitsuhashi T., Tennyson G.E., Nikodem V.M. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc. Natl. Acad. Sci. USA. 1988;85:5804–5808. doi: 10.1073/pnas.85.16.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai A., Nakai A., DeGroot L.J. Expression of three forms of thyroid hormone receptor in human tissues. Mol. Endocrinol. 1989;3:392–399. doi: 10.1210/mend-3-2-392. [DOI] [PubMed] [Google Scholar]
- 44.Pascual A., Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim. Biophys. Acta. 2013;1830:3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Perlmann T., Rangarajan P.N., Umesono K., Evans R.M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993;7:1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- 46.Heublein S., Mayr D., Meindl A., Angele M., Gallwas J., Jeschke U., Ditsch N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PLoS ONE. 2015;10:e0127072. doi: 10.1371/journal.pone.0127072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S., Roman J. Peroxisome proliferator-activated receptor gamma: A novel target for cancer therapeutics? Anticancer Drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 48.Remmele W., Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 49.Joseph C., Al-Izzi S., Alsaleem M., Kurozumi S., Toss M.S., Arshad M., Goh F.Q., Alshankyty I.M., Aleskandarany M.A., Ali S., et al. Retinoid X receptor gamma (RXRG) is an independent prognostic biomarker in ER-positive invasive breast cancer. Br. J. Cancer. 2019;121:776–785. doi: 10.1038/s41416-019-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roszer T., Menendez-Gutierrez M.P., Cedenilla M., Ricote M. Retinoid X receptors in macrophage biology. Trends Endocrinol. Metab. 2013;24:460–468. doi: 10.1016/j.tem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Leal A.S., Zydeck K., Carapellucci S., Reich L.A., Zhang D., Moerland J.A., Sporn M.B., Liby K.T. Retinoid X receptor agonist LG100268 modulates the immune microenvironment in preclinical breast cancer models. NPJ Breast Cancer. 2019;5:39. doi: 10.1038/s41523-019-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nunez V., Alameda D., Rico D., Mota R., Gonzalo P., Cedenilla M., Fischer T., Bosca L., Glass C.K., Arroyo A.G., et al. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc. Natl. Acad. Sci. USA. 2010;107:10626–10631. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalra R.S., Bapat S.A. Expression proteomics predicts loss of RXR-gamma during progression of epithelial ovarian cancer. PLoS ONE. 2013;8:e70398. doi: 10.1371/journal.pone.0070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S.M., Lee J.Y., Choi J.E., Lee S.Y., Park J.Y., Kim D.S. Epigenetic inactivation of retinoid X receptor genes in non-small cell lung cancer and the relationship with clinicopathologic features. Cancer Genet. Cytogenet. 2010;197:39–45. doi: 10.1016/j.cancergencyto.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Liby K., Rendi M., Suh N., Royce D.B., Risingsong R., Williams C.R., Lamph W., Labrie F., Krajewski S., Xu X., et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin. Cancer Res. 2006;12:5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 56.Wu K., Kim H.T., Rodriquez J.L., Hilsenbeck S.G., Mohsin S.K., Xu X.C., Lamph W.W., Kuhn J.G., Green J.E., Brown P.H. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol. Biomark. Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 57.Kong G., Kim H.T., Wu K., DeNardo D., Hilsenbeck S.G., Xu X.C., Lamph W.W., Bissonnette R., Dannenberg A.J., Brown P.H. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: Implications for molecular-based chemoprevention. Cancer Res. 2005;65:3462–3469. doi: 10.1158/0008-5472.CAN-03-2912. [DOI] [PubMed] [Google Scholar]
- 58.Wu K., DuPre E., Kim H., Tin U.C., Bissonnette R.P., Lamph W.W., Brown P.H. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res. Treat. 2006;96:147–157. doi: 10.1007/s10549-005-9071-1. [DOI] [PubMed] [Google Scholar]
- 59.Esteva F.J., Glaspy J., Baidas S., Laufman L., Hutchins L., Dickler M., Tripathy D., Cohen R., DeMichele A., Yocum R.C., et al. Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J. Clin. Oncol. 2003;21:999–1006. doi: 10.1200/JCO.2003.05.068. [DOI] [PubMed] [Google Scholar]
- 60.Alvarado-Pisani A.R., Chacon R.S., Betancourt L.J., Lopez-Herrera L. Thyroid hormone receptors in human breast cancer: Effect of thyroxine administration. Anticancer Res. 1986;6:1347–1351. [PubMed] [Google Scholar]
- 61.Cestari S.H., Figueiredo N.B., Conde S.J., Clara S., Katayama M.L., Padovani C.R., Brentani M.M., Nogueira C.R. Influence of estradiol and triiodothyronine on breast cancer cell lines proliferation and expression of estrogen and thyroid hormone receptors. Arq. Bras. Endocrinol. Metab. 2009;53:859–864. doi: 10.1590/S0004-27302009000700010. [DOI] [PubMed] [Google Scholar]
- 62.Hall L.C., Salazar E.P., Kane S.R., Liu N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid. Biochem. Mol. Biol. 2008;109:57–66. doi: 10.1016/j.jsbmb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Jerzak K.J., Cockburn J., Pond G.R., Pritchard K.I., Narod S.A., Dhesy-Thind S.K., Bane A. Thyroid hormone receptor alpha in breast cancer: Prognostic and therapeutic implications. Breast Cancer Res. Treat. 2015;149:293–301. doi: 10.1007/s10549-014-3235-9. [DOI] [PubMed] [Google Scholar]
- 64.Conde I., Paniagua R., Zamora J., Blanquez M.J., Fraile B., Ruiz A., Arenas M.I. Influence of thyroid hormone receptors on breast cancer cell proliferation. Ann. Oncol. 2006;17:60–64. doi: 10.1093/annonc/mdj040. [DOI] [PubMed] [Google Scholar]
- 65.Silva J.M., Dominguez G., Gonzalez-Sancho J.M., Garcia J.M., Silva J., Garcia-Andrade C., Navarro A., Munoz A., Bonilla F. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21:4307–4316. doi: 10.1038/sj.onc.1205534. [DOI] [PubMed] [Google Scholar]
- 66.Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S., et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 67.Li Z., Meng Z.H., Chandrasekaran R., Kuo W.L., Collins C.C., Gray J.W., Dairkee S.H. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002;62:1939–1943. [PubMed] [Google Scholar]
- 68.Ditsch N., Toth B., Himsl I., Lenhard M., Ochsenkuhn R., Friese K., Mayr D., Jeschke U. Thyroid hormone receptor (TR)alpha and TRbeta expression in breast cancer. Histol. Histopathol. 2013;28:227–237. doi: 10.14670/HH-28.227. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro R.C., Apriletti J.W., Wagner R.L., Feng W., Kushner P.J., Nilsson S., Scanlan T.S., West B.L., Fletterick R.J., Baxter J.D. X-ray crystallographic and functional studies of thyroid hormone receptor. J. Steroid Biochem. Mol. Biol. 1998;65:133–141. doi: 10.1016/S0960-0760(98)00029-6. [DOI] [PubMed] [Google Scholar]
- 70.Lazar J., Desvergne B., Zimmerman E.C., Zimmer D.B., Magnuson M.A., Nikodem V.M. A role for intronic sequences on expression of thyroid hormone receptor alpha gene. J. Biol. Chem. 1994;269:20352–20359. doi: 10.1016/S0021-9258(17)31999-3. [DOI] [PubMed] [Google Scholar]
- 71.Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988;334:539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- 72.Hercbergs A., Johnson R.E., Ashur-Fabian O., Garfield D.H., Davis P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist. 2015;20:72–76. doi: 10.1634/theoncologist.2014-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hercbergs A.A., Goyal L.K., Suh J.H., Lee S., Reddy C.A., Cohen B.H., Stevens G.H., Reddy S.K., Peereboom D.M., Elson P.J., et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003;23:617–626. [PubMed] [Google Scholar]
- 74.Dinda S., Sanchez A., Moudgil V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene. 2002;21:761–768. doi: 10.1038/sj.onc.1205136. [DOI] [PubMed] [Google Scholar]
- 75.Sabnis G.J., Goloubeva O., Chumsri S., Nguyen N., Sukumar S., Brodie A.M. Functional activation of the estrogen receptor-alpha and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res. 2011;71:1893–1903. doi: 10.1158/0008-5472.CAN-10-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cserni G., Chmielik E., Cserni B., Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472:697–703. doi: 10.1007/s00428-018-2301-9. [DOI] [PubMed] [Google Scholar]
- 77.Hortobagyi G.N., Edge S.B., Giuliano A. New and Important Changes in the TNM Staging System for Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:457–467. doi: 10.1200/EDBK_201313. [DOI] [PubMed] [Google Scholar]
- 78.Elston C.W., Ellis I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 2002;41:151–152. doi: 10.1046/j.1365-2559.2002.14691.x. [DOI] [PubMed] [Google Scholar]
- 79.Ditsch N., Mayr D., Lenhard M., Strauss C., Vodermaier A., Gallwas J., Stoeckl D., Graeser M., Weissenbacher T., Friese K., et al. Correlation of thyroid hormone, retinoid X, peroxisome proliferator-activated, vitamin D and oestrogen/progesterone receptors in breast carcinoma. Oncol. Lett. 2012;4:665–671. doi: 10.3892/ol.2012.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are accessible on request from the authors.