Abstract
Objective
To evaluate the association between baseline apathy and probable incident dementia in a population-based sample of community-dwelling older adults.
Methods
We studied 2,018 white and black community-dwelling older adults from the Health, Aging, and Body Composition (Health ABC) study. We measured apathy at year 6 (our study baseline) with the modified Apathy Evaluation Scale and divided participants into tertiles based on low, moderate, or severe apathy symptoms. Incident dementia was ascertained over 9 years by dementia medication use, hospital records, or clinically relevant cognitive decline on global cognition. We examined the association between apathy and probable incident dementia using a Cox proportional hazards model adjusting for demographics, cardiovascular risk factors, APOE4 status, and depressed mood. We also evaluated the association between the apathy group and cognitive change (as measured by the modified Mini-Mental State Examination and Digit Symbol Substitution Test over 5 years) using linear mixed effects models.
Results
Over 9 years of follow-up, 381 participants developed probable dementia. Severe apathy was associated with an increased risk of dementia compared to low apathy (25% vs 14%) in unadjusted (hazard ratio [HR] 1.9, 95% confidence interval [CI] 1.5–2.5) and adjusted models (HR 1.7, 95% CI 1.3–2.2). Greater apathy was associated with worse cognitive score at baseline, but not rate of change over time.
Conclusion
In a diverse cohort of community-dwelling adults, apathy was associated with increased risk of developing probable dementia. This study provides novel evidence for apathy as a prodrome of dementia.
The prevalence of dementia is increasing1 and there is growing interest in identifying preclinical markers of cognitive decline to better understand an individual's risk. Neuropsychiatric symptoms (NPS) are common across the spectrum of cognitive function in older adults, affecting up to half of patients with mild cognitive impairment (MCI) and nearly all patients with dementia over the course of their disease.2–4 NPS have prognostic value in predicting accelerated disease progression5 and functional decline.6,7 In addition, both early-life8,9 and late-life depression10–12 have been established as important predictors of the incidence of dementia. Given the clinical relevance of NPS, experts have proposed the syndrome of mild behavioral impairment as an entity that precedes dementia onset.13 However, few neuropsychiatric symptoms besides depression have been evaluated as independent predictors of cognitive decline in prospective studies.
Apathy, defined as decreased motivation and goal-directed behavior, is the most prevalent neuropsychiatric symptom among the dementia subtypes.14 Apathy is correlated with depression but is a distinct entity with unique neuroanatomic correlates in the dorsolateral prefrontal cortex and associated subregions in the basal ganglia.15,16 Despite some overlap in symptoms, appropriate diagnostic criteria can clinically separate apathy from depression.17 Indeed, approximately one third of patients with dementia and apathy do not exhibit signs of comorbid depression.18
Previous cohort studies suggest that patients with MCI and apathy have a higher incident risk of dementia.19–24 Apathy has therefore been flagged by the National Institute on Aging as a highly informative neuropsychiatric risk state.25 However, existing studies have primarily been conducted in participants with preexisting MCI and are limited by small sample size,19,20,22 short follow-up times,19,20,23 or a lack of generalizability.26 There are no large studies that have investigated apathy as an independent risk factor for or prodrome of dementia in a diverse sample of cognitively normal older adults. The goal of our study was to evaluate the hypothesis that apathy is a prodrome of incident dementia in a population-based sample of community-dwelling elders.
Methods
Standard protocol approvals, registrations, and patient consents
All participants signed a written informed consent, approved by the institutional review boards at each clinical site and the coordinating site. Full details of the Health, Aging, and Body Composition (Health ABC) study have been published.27
Population
We studied participants from the Health ABC study, a prospective cohort study of community-dwelling white and black older adults. The study was based in Memphis, Tennessee, and Pittsburgh, Pennsylvania, with the coordinating center in San Francisco, California. Potential participants were identified and contacted based on a random sample of Medicare-eligible adults (age 70–79) within predesignated zip codes. Participants were excluded if they had mobility or functional limitations, a life-threatening diagnosis such as cancer, or plans to leave the area within 3 years.
A total of 3,075 adults were enrolled from May 1997 to June 1998. For this study, we excluded participants who did not undergo a year 6 visit (456 participants), those who were identified to have dementia prior to our study baseline at year 6 (131 participants), and those who did not complete the apathy evaluation (465 participants). A total of 2,018 participants were included in our analytic cohort.
Measures
Apathy
We measured apathy with a modified version of the Apathy Evaluation Scale, a well-validated scale with the ability to discriminate apathy from depression and anxiety.28 The 18-question Apathy Evaluation Scale was adapted to 5 questions based on expert consensus at the time of study design (table 1). Answers to questions were based on a Likert scale and scored between 0 (“Rarely or none of the time”) and 3 (“Most or all of the time”). The total score ranges between 0 and 15, with 0 representing low apathy and 15 representing high apathy. Questions were administered by trained study staff at an in-person clinic visit using standardized scripts at year 6, our study baseline. We divided participants into tertiles based on the study sample, corresponding to low, moderate, or severe apathy.
Table 1.
Questions and response options for the modified Apathy Evaluation Scale

Cognition
Participants underwent cognitive evaluation with the modified Mini-Mental State Examination (3MS) and the Digit Symbol Substitution Test (DSST) at years 5, 8, and 10. The 3MS assesses orientation, concentration, language, praxis, immediate memory, and delayed memory to yield a score between 0 and 100.29 It is highly sensitive in detecting cognitive decline compared to other standard measurements.30 The DSST assesses attention, processing speed, visuospatial function, and working memory.
Incident dementia
We determined incident dementia with a previously published algorithm incorporating dementia medication use, hospital records, or significant global cognitive decline.31 Health ABC staff surveyed participants every 6 months regarding occurrence of hospitalizations, reviewed records associated with hospitalization, and recorded participants' home medications at each visit. The date of dementia incidence was defined as the first date that a participant met any of the following criteria: (1) occurrence of a hospitalization with dementia documented as a primary or secondary diagnosis; (2) identification of a prescription for a dementia medication (galantamine, memantine, donepezil, rivastigmine, or tacrine); or (3) clinically significant decline in cognition (>1.5 race-specific SD) from baseline to the last available cognitive score.
Other variables
Age, race, sex, and educational level were self-reported at baseline. Depressed mood was measured by the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item measure with a cutoff of 15 to identify severe depressive symptoms.32 Hypertension was identified by self-report of diagnosis, use of any antihypertensive medication, or measured systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg. History of myocardial infarction, stroke, or TIA was determined by either a physician or self-reported diagnosis. Body mass index (kg/m2) was calculated from direct height and weight measurements recorded at baseline. Participants self-reported use of cigarettes and alcohol. APOE genotype was determined by single nucleotide polymorphism analyses.
Statistical analysis
We used χ2 tests and analysis of variance to evaluate whether participant characteristics differed between those reporting low, moderate, or severe apathy symptoms. To examine the association between apathy and probable incident dementia, we plotted Kaplan-Meier survival curves and computed Cox proportional hazards regression models. The time to event was calculated from baseline at year 6 until the date of dementia diagnosis during follow-up. Individuals without dementia were censored at the last available date of contact. We first analyzed the association between apathy and probable incident dementia (model 1) and then adjusted for demographics, education, history of myocardial infarction, history of stroke, hypertension, cigarette smoking, and APOE4 status (model 2). In order to assess independence from depression, we then separately adjusted for depressed mood (model 3). We also tested for interactions between apathy tertile and sex, race, and APOE4 status. To evaluate the robustness of our findings, we conducted sensitivity analyses excluding participants diagnosed with dementia only according to the neuropsychological testing criterion, participants with baseline depressed mood (CES-D score >15), and participants with baseline probable MCI.
Additional analyses were performed to evaluate change in cognition over time. We used linear mixed-effects regression models to investigate the association between apathy groups and change in cognition over follow-up from year 5 (the closest measurement to our study baseline) to year 10. Multivariable models were adjusted for demographics, education, history of myocardial infarction, history of stroke, hypertension, cigarette smoking, and depressed mood. All analyses were conducted in Stata (StataCorp, College Station, TX). Statistical significance was set at p < 0.05.
Data availability
Data not published within the article are available in a public repository. Anonymized data will be shared by request from any qualified investigator.
Results
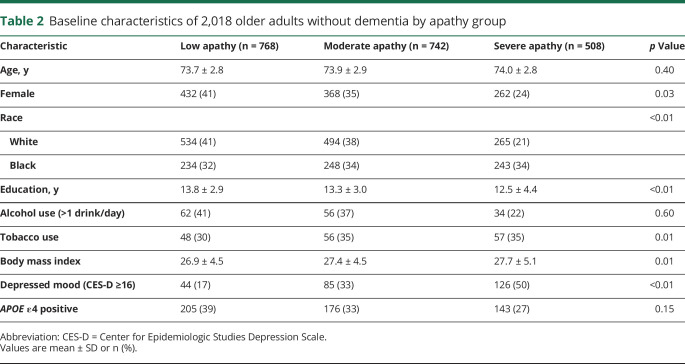
Demographic characteristics are summarized in table 2. Of the 2,018 participants without dementia at baseline, the mean age was 73.9 years (SD 2.8). Approximately one third of participants were black (35.9%) and two thirds were white (64.1%). Slightly more than half (52.3%) of the cohort was female. Participants reported an average of 13.3 years (SD 3.4) of education. Participants were followed for a mean of 5.8 years (SD 3.0) until they were diagnosed with probable dementia or were censored. Participants who completed the apathy measure were more likely to be higher educated (F = 44.4, p < 0.001) and white (Pearson χ2 = 37.0, p < 0.001). There was no difference in likelihood of completion of the apathy measure by sex, APOE4 status, or depressed mood.
Table 2.
Baseline characteristics of 2,018 older adults without dementia by apathy group
There were 768 (38%) participants in the low apathy group, 742 (37%) participants in the moderate apathy group, and 508 (25%) participants in the severe apathy group. Participants with greater apathy at baseline were significantly more likely to be male, black, and less educated. They were also more likely to have a higher body mass index and report a history of cigarette smoking. There were 255 participants in the cohort that met criteria for clinically depressed mood according to the CES-D. Half of these individuals (126/255) reported symptoms of severe apathy, compared to 33% (85/255) that reported moderate apathy and 17% (44/255) that reported low apathy.
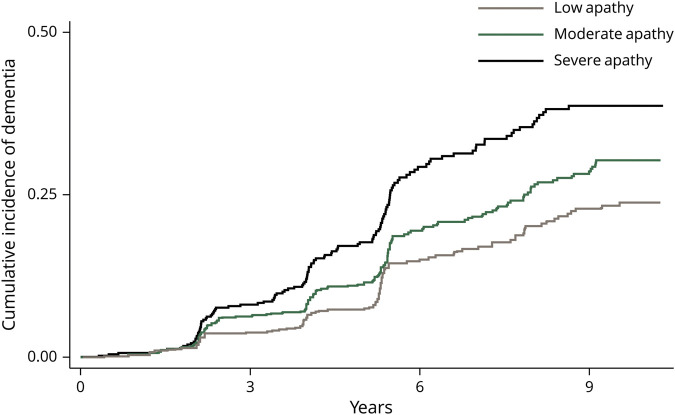
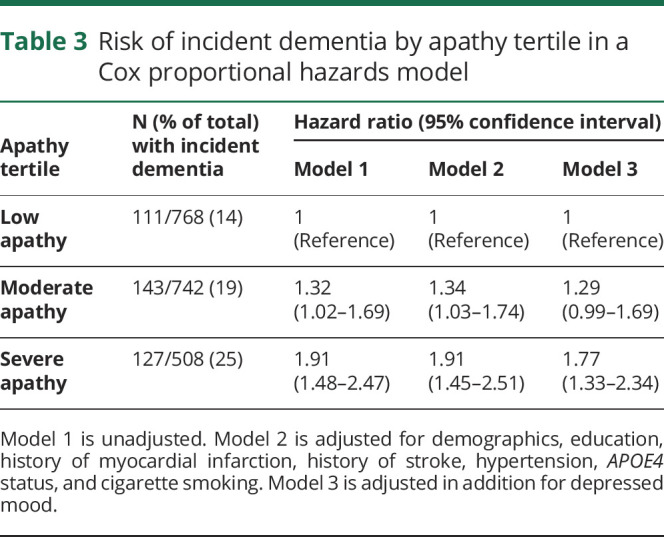
Of 2,018 participants, 381 individuals developed probable dementia during the follow-up period. Twenty-nine (8%) were diagnosed by medication prescription only, 151 (40%) were diagnosed according to hospital records only, 56 (15%) were identified by a >1.5 SD change in the 3MS only, and 129 (34%) were diagnosed based on a combination of these findings. Participants developed dementia per our criteria an average of 4.6 years (SD 2.1) after the apathy assessment. In the severe apathy group, 25% of participants (127/509) developed probable incident dementia, compared to 14% (111/660) of those with low apathy. Kaplan-Meier survival curves by apathy group (figure 1) show that higher apathy was associated with higher risk of probable dementia in a graded fashion (p < 0.0001 by log-rank test). Table 3 summarizes the results of the Cox proportional hazards regression models. Compared to those with low apathy, participants with severe apathy were approximately twice as likely to develop probable dementia (hazard ratio [HR] 1.9, 95% CI 1.5–2.5, p < 0.001), an association that remained robust in the fully adjusted model (adjusted HR 1.8, 95% CI 1.3–2.3, p < 0.001). Participants reporting moderate apathy at baseline were 30% more likely to develop probable dementia compared to those with low apathy over the study period (HR 1.3, 95% CI 1.0–1.7, p = 0.03), but this association was no longer significant in the fully adjusted model (adjusted HR 1.3, CI 1.0–1.7, p = 0.06). There were no significant interactions between apathy group and sex, race, or APOE4 status (p > 0.05 for all).
Figure 1. Association between apathy and incident dementia among 2,018 older adults.
Kaplan-Meier curves show dementia incidence over 9 years by apathy tertile (p < 0.0001 by log-rank test).
Table 3.
Risk of incident dementia by apathy tertile in a Cox proportional hazards model
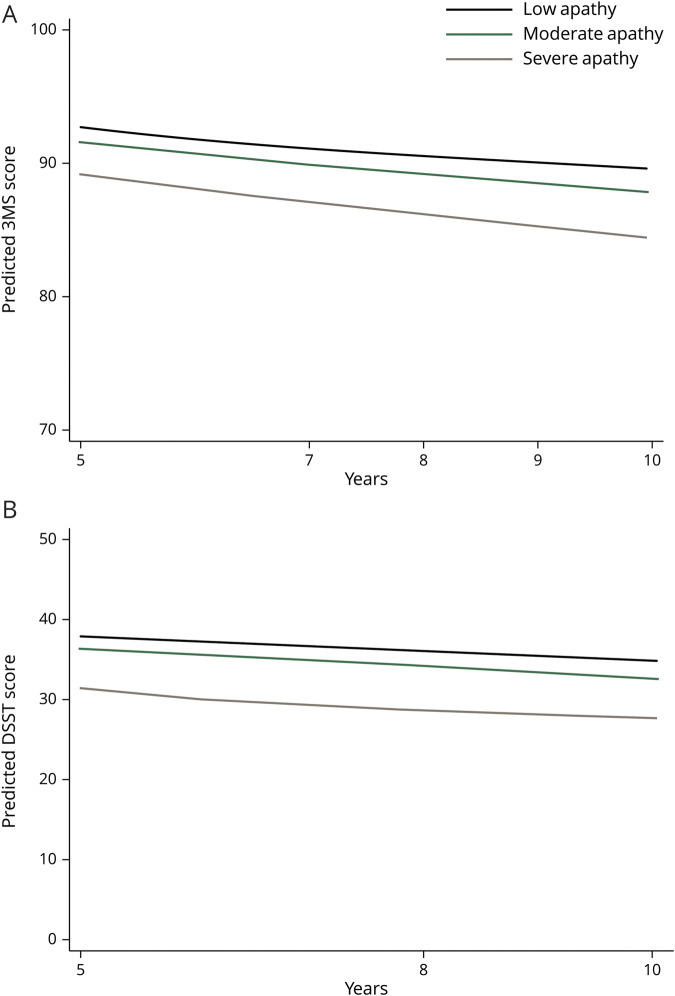
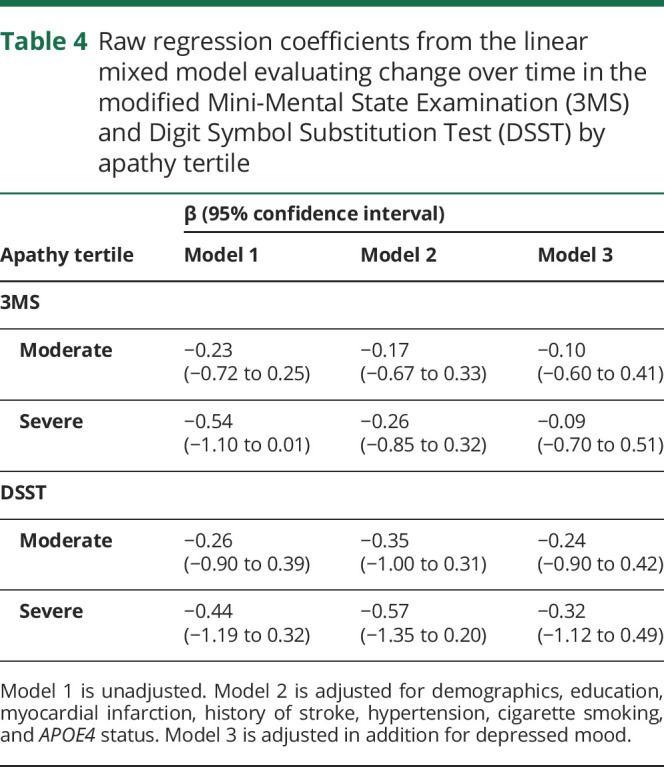
Additional analyses examined whether the apathy groups were associated with cognition. Compared to the low apathy group, the severe apathy group performed 1.6 points lower on the DSST (95% CI 0.2–2.9, p = 0.029) and 0.9 points lower on the 3MS at baseline (95% CI 0.1–1.6, p = 0.019) in fully adjusted linear regression models. However, there was no association observed between the apathy groups and change in cognition over time in unadjusted or adjusted linear mixed-effects regression models (figure 2, table 4).
Figure 2. Modified Mini-Mental State Examination (3MS) and Digit Symbol Substitution Test (DSST) over time by apathy tertile, implied by linear mixed effects model.
(A) 3MS. (B) DSST. Models included demographics, education, history of myocardial infarction, history of stroke, hypertension, cigarette smoking, APOE4, and depressed mood.
Table 4.
Raw regression coefficients from the linear mixed model evaluating change over time in the modified Mini-Mental State Examination (3MS) and Digit Symbol Substitution Test (DSST) by apathy tertile
We conducted additional sensitivity analyses. We repeated our Cox proportional hazard model sequentially excluding patients diagnosed with dementia only according to change in 3MS score (n = 56) or participants with baseline probable cognitive impairment (1.0–1.5 race-specific SD below the mean 3MS score). We also repeated analyses excluding the 258 patients with baseline depressed mood as measured by the CES-D. Results were very similar to our primary analyses and remained statistically significant.
Discussion
We found that community-dwelling older adults who report greater symptoms of apathy are approximately 80% more likely to develop probable dementia compared to those with few symptoms. Our study provides novel evidence for apathy as a prodrome of dementia.
Our findings are supported by prior studies showing that apathy increases the risk of probable dementia approximately twofold in individuals with MCI,19–24 but extends this result to a population-based cohort of older adults without dementia. The majority of prior studies were conducted in patients sampled from memory clinics21,33 or based on preexisting diagnoses of cognitive impairment.13–16 These restrictions on patient cohorts and shorter follow-up time limits the ability to identify whether apathy was a prodromal symptom in the pathway of neurodegeneration, causal risk factor, or reaction to the diagnosis of dementia.34 A prior meta-analysis suggested that apathy is prodromal because the association between apathy and dementia in patients with MCI diminished with longer follow-up, but this had not been rigorously evaluated.35 The few existing studies in cognitively normal older adults had conflicting results regarding the risk of dementia conferred by apathy. One study found a 7-fold increase in the risk of Alzheimer dementia26 and 2 others identified progression to MCI but not dementia over a short time frame.19,36 In addition, existing studies were conducted in primarily white patients drawn from academic centers, whereas our study was 36% nonwhite and is more generalizable to the US population.
Our results support the hypothesis that apathy is a prodrome to dementia, which is in line with early studies evaluating the neurobiology of apathy in neurodegenerative conditions. In individuals with dementia, symptoms of apathy correlate with increased prefrontal tau and amyloid burden.37,38 Neuroimaging studies also demonstrate that loss of white matter connectivity can contribute significantly to symptoms of apathy.39,40 In adults with varied cognition, the posterior cingulate and inferior temporal cortex exhibit atrophy early in apathy development, with progression to involve the anterior cingulate, inferior frontal cortex, and insula.41,42 There is conflicting evidence regarding the genetic underpinnings of apathy, with 2 studies finding an increased risk of apathy in APOE4 carriers43 and others finding no association.44,45 In our cohort, we did not find a significant interaction between APOE4 status and apathy in predicting dementia. While it is possible that apathy also represents a causal risk factor for dementia, likely mediated by social withdrawal, our study adds to the growing body of evidence that it is a prodromal symptom.
In our study, apathy did not predict greater cognitive decline as measured by the 3MS and DSST, suggesting that apathy may be less helpful in predicting cognitive decline below the dementia threshold. However, prior studies found significant short-term cognitive decline after identification of apathy.7 In our cohort, apathy was not measured until year 6 and we excluded participants who developed dementia prior to this, so the patients with more rapid cognitive decline were excluded from the analysis. Alternatively, prodromal apathy may represent selective vulnerability of frontal circuits that leads to changes less readily detectable on some cognitive tests. Individuals with dementia and apathy have previously been shown to have more impairment in multitasking, selective attention, and cognitive flexibility.46 Our neuropsychological measures evaluated executive function, but are less likely to identify disinhibition, impaired judgement, and other behavioral changes that nonetheless significantly impact function. The presence of apathy is distressing to family and interferes substantially with activities of daily living, so this symptom may also increase the likelihood that a patient is diagnosed with dementia by a clinician even without a statistically significant difference in neuropsychological performance.
Strengths of our study include the diversity of our cohort, longer follow-up time in a sample of patients who are not cognitively impaired at baseline, and the ability to control for depressed mood and a number of cardiovascular risk factors. Our sample was less subject to referral bias that affects most studies conducted in memory clinics, but is subject to the healthy volunteer bias. However, population-based studies are particularly important when studying apathy, a symptom that may cause individuals to minimize contact with the health care system. Limitations include our use of an algorithm to diagnose dementia, which may not be as sensitive as an in-depth clinical evaluation and did not enable us to diagnose dementia subtypes. We did not have data available on hospital-acquired delirium to investigate how this could be associated with dementia. As delirium is a transient state and variably coded, surveillance of delirium is challenging using hospital data. Second, apathy was self-reported in this study in a population that may be experiencing behavioral changes with variable levels of insight. However, self-report is a common form of clinician assessment of behavioral symptoms and is more reliable in cognitively normal adults.42 Sensitivity analyses excluding patients with baseline probable MCI showed no substantial difference in HRs. Third, the literature on depression as a predictor of dementia suggests that the timing of onset,9 severity of symptoms,12 and trajectory47 are all important modifiers between depression and dementia risk. Our evaluation of these modifiers was limited by the lack of repeated evaluations or evaluation of symptoms earlier in life, though apathy is more likely to be a persistent state.48,49
We found that apathy is independently associated with increased risk of future probable dementia. As apathy is typically clinically apparent and highly distressing to family, a brief evaluation of apathy may be a helpful tool to identify older adults at increased risk of dementia. Neuroimaging and CSF biomarkers are promising new techniques in identifying prodromal dementia,50 but they are currently under development and may not be available in underresourced health care systems. Future research is needed to further understand the neurobiology of apathy and the role of apathy screening in identifying individuals who would benefit from early reduction of modifiable risk factors or consideration for clinical trials.
Acknowledgment
The authors thank the Center for Population Brain Health for their time and feedback on this project.
Glossary
- 3MS
modified Mini-Mental State Examination
- CES-D
Center for Epidemiologic Studies Depression Scale
- DSST
Digit Symbol Substitution Test
- Health ABC
Health, Aging, and Body Composition study
- HR
hazard ratio
- MCI
mild cognitive impairment
- NPS
neuropsychiatric symptoms
Appendix. Authors

Study funding
This project is supported by the NIA through grant number K24 AG-31155.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Prince MJ, Guerchet M, Prina M. The Global Impact of Dementia 2013-2050: Policy Brief for Heads of Government. London, UK: Alzheimer's Disease International; 2013. [Google Scholar]
- 2.Mortby ME, Burns R, Eramudugolla R, Ismail Z, Anstey KJ. Neuropsychiatric symptoms and cognitive impairment: understanding the importance of co-morbid symptoms. J Alzheimers Dis 2017;59:141–153. [DOI] [PubMed] [Google Scholar]
- 3.Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int Psychogeriatr 2018;30:221–232. [DOI] [PubMed] [Google Scholar]
- 4.Peters ME, Rosenberg PB, Steinberg M, et al. Prevalence of neuropsychiatric symptoms in CIND and its subtypes: the Cache County Study. Am J Geriatr Psychiatry 2012;20:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters ME, Schwartz S, Han D, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer's dementia and death: the Cache County Dementia Progression Study. Am J Psychiatry 2015;172:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rog LA, Park LQ, Harvey DJ, Huang CJ, Mackin S, Farias ST. The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. Clin Neuropsychol 2014;28:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke DE, Ko JY, Lyketsos C, Rebok GW, Eaton WW. Apathy and cognitive and functional decline in community-dwelling older adults: results from the Baltimore ECA longitudinal study. Int Psychogeriatr 2010;22:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012;69:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010;75:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry 2012;20:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 2010;75:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002;59:364–370. [DOI] [PubMed] [Google Scholar]
- 13.Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Demen 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez Martinez M, Castro Flores J, Perez de las Heras S, Mandaluniz Lekumberri A, Gordejuela Menocal M, Zarranz Imirizaldu JJ. Prevalence of neuropsychiatric symptoms in elderly patients with dementia in Mungialde County (Basque Country, Spain). Demen Geriatr Cogn Disord 2008;25:103–108. [DOI] [PubMed] [Google Scholar]
- 15.Holthoff VA, Beuthien-Baumann B, Kalbe E, et al. Regional cerebral metabolism in early Alzheimer's disease with clinically significant apathy or depression. Biol Psychiatry 2005;57:412–421. [DOI] [PubMed] [Google Scholar]
- 16.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 2006;16:916–928. [DOI] [PubMed] [Google Scholar]
- 17.Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry 2011;26:158–165. [DOI] [PubMed] [Google Scholar]
- 18.Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer's disease. Am J Psychiatry 2001;158:872–877. [DOI] [PubMed] [Google Scholar]
- 19.Brodaty H, Heffernan M, Draper B, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis 2012;31:411–420. [DOI] [PubMed] [Google Scholar]
- 20.Chan WC, Lam LC, Tam CW, et al. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing 2011;40:30–35. [DOI] [PubMed] [Google Scholar]
- 21.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis 2010;20:175–183. [DOI] [PubMed] [Google Scholar]
- 22.Pink A, Stokin GB, Bartley MM, et al. Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology 2015;84:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert PH, Berr C, Volteau M, et al. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer's disease: a one-year follow-up study. Clin Neurol Neurosurg 2006;108:733–736. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013;21:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's disease. Alzheimers Demen 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke SL, Maramaldi P, Cadet T, Kukull W. Neuropsychiatric symptoms and apolipoprotein E: associations with eventual Alzheimer's disease development. Arch Gerontol Geriatr 2016;65:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the health, aging, and body composition study. J Gerontol B Psychol Sci Soc Sci 2002;57:S247–S256. [DOI] [PubMed] [Google Scholar]
- 28.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 30.Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JW, Jr. Screening for cognitive impairment: comparing the performance of four instruments in primary care. J Am Geriatr Soc 2012;60:1027–1036. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994;10:77–84. [PubMed] [Google Scholar]
- 33.Richard E, Schmand B, Eikelenboom P, et al. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer's disease in non-depressed subjects. Demen Geriatr Cogn Disord 2012;33:204–209. [DOI] [PubMed] [Google Scholar]
- 34.Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimers Demen 2013;9:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dalen JW, van Wanrooij LL, Moll van Charante EP, Brayne C, van Gool WA, Richard E. Association of apathy with risk of incident dementia: a systematic review and meta-analysis. JAMA psychiatry 2018;75:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014;171:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori T, Shimada H, Shinotoh H, et al. Apathy correlates with prefrontal amyloid beta deposition in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2014;85:449–455. [DOI] [PubMed] [Google Scholar]
- 38.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer's disease. Demen Geriatr Cogn Disord 2006;21:144–147. [DOI] [PubMed] [Google Scholar]
- 39.Hahn C, Lim HK, Won WY, Ahn KJ, Jung WS, Lee CU. Apathy and white matter integrity in Alzheimer's disease: a whole brain analysis with tract-based spatial statistics. PLoS One 2013;8:e53493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torso M, Serra L, Giulietti G, et al. Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer's disease. PLoS One 2015;10:e0124998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donovan NJ, Wadsworth LP, Lorius N, et al. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry 2014;22:1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guercio BJ, Donovan NJ, Munro CE, et al. The apathy evaluation scale: a comparison of subject, informant, and clinician report in cognitively normal elderly and mild cognitive impairment. J Alzheimers Dis 2015;47:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Onofrio G, Panza F, Seripa D, et al. The APOE polymorphism in Alzheimer's disease patients with neuropsychiatric symptoms and syndromes. Int J Geriatr Psychiatry 2011;26:1062–1070. [DOI] [PubMed] [Google Scholar]
- 44.Del Prete M, Spaccavento S, Craca A, Fiore P, Angelelli P. Neuropsychiatric symptoms and the APOE genotype in Alzheimer's disease. Neurol Sci 2009;30:367–373. [DOI] [PubMed] [Google Scholar]
- 45.Borroni B, Grassi M, Agosti C, et al. Genetic correlates of behavioral endophenotypes in Alzheimer disease: role of COMT, 5-HTTLPR and APOE polymorphisms. Neurobiol Aging 2006;27:1595–1603. [DOI] [PubMed] [Google Scholar]
- 46.Esposito F, Rochat L, Van der Linden AC, et al. Apathy and executive dysfunction in Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24:131–137. [DOI] [PubMed] [Google Scholar]
- 47.Mirza SS, Wolters FJ, Swanson SA, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 2016;3:628–635. [DOI] [PubMed] [Google Scholar]
- 48.Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FR. The course of neuropsychiatric symptoms in dementia: part I: findings from the two-year longitudinal Maasbed study. Int J Geriatr Psychiatry 2005;20:523–530. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2004;19:19–26. [DOI] [PubMed] [Google Scholar]
- 50.Richard E, Schmand BA, Eikelenboom P, Van Gool WA. MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open 2013;3:e002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not published within the article are available in a public repository. Anonymized data will be shared by request from any qualified investigator.