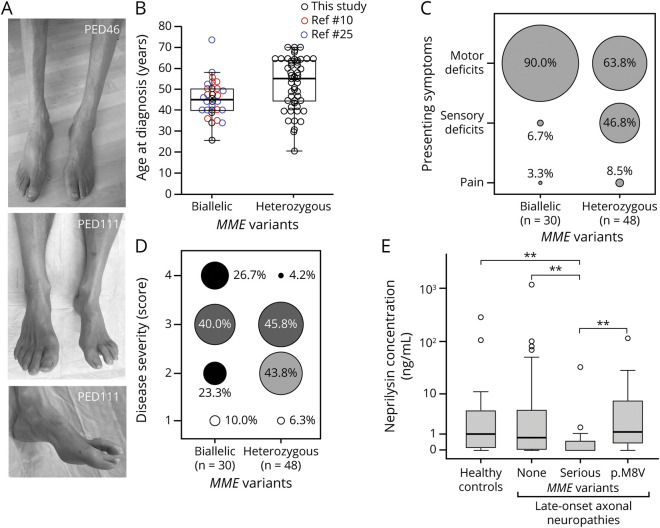
Figure 3. MME-related late-onset neuropathies.
(A) Distal muscle atrophy and pes cavus deformity in individuals with heterozygous p.Trp24* (PED46) and heterozygous p.Thr100Profs*11 (PED111) MME variants. (B) Comparison of the age at disease onset in cases with autosomal-recessive and assumed autosomal-dominant inheritance of MME variants reported in this study and previous studies.10,25 Median, quartiles, and whiskers corresponding to 1.5 times the interquartile range (IQR) are shown. (C) Distribution of presenting symptoms in cases with autosomal-recessive and assumed autosomal-dominant inheritance of MME variants. Cases with biallelic variants also include the series of patients reported by other studies.2,10,25 Diameters of the circles correspond to the proportion of cases with the respective symptom. (D) Distribution of disease severity scores from 1 (mild) to 4 (very severe) in cases with autosomal-recessive and assumed autosomal-dominant inheritance of MME variants. Cases with biallelic variants also include the series of patients reported by previous studies.10,25 Diameters of the circles correspond to the proportion of cases with the respective severity score. (E) Boxplots comparing neprilysin levels in EDTA plasma obtained from healthy controls (n = 22), patients with late-onset neuropathy without MME variants (n = 34), those with serious MME variants (n = 15), and patients with the p.Met8Val low-frequency polymorphism (n = 9). Outliers (3× IQR) are depicted as open circles and were not included in the statistical analyses. **p < 0.01 (Kruskal-Wallis test with Dunn post hoc test and Bonferroni correction).