Table 2.
Biologically Important Buffering Reactions and Their pKas
| Reaction | pKa |
|---|---|
| H2O ⇌ OH− + H+ | 14.0 |
| H2O + H2O ⇌ OH− + H3O+ | 14.0 |
| CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3− | 6.1 |
| CO2 + H2O ⇌ H2CO3 | 3.6 |
| H2CO3 ⇌ H+ + HCO3− | 6.3 |
| HCO3− ⇌ H+ + CO32− | 10.32 |
| NH3 + H+ ↔ NH4+ | 9.25 |
| H-lactate (CH3CH(OH)CO2H) ⇌ H+ + CH3CH(OH)CO2 | 3.86 |
| H-pyruvate (CH3COCOOH) ⇌ H+ + pyruvate− (CH3COCOO−) | 2.50 |
| H-butyrate (CH3CH2CH2COOH) ⇌ H+ + butyrate− (CH3CH2CH2COOH−) | 4.82 |
| H-propionate (CH3CH2COOH) ⇌ H+ + propionate− (CH3CH2COOH−) | 4.88 |
| H-acetate (CH3COOH) ⇌ H+ + acetate− (CH3COOH−) | 4.76 |
| H3PO4 ⇌ H2PO4− + H+ | 2.14 |
| H2PO4− ⇌ HPO42−+H+ | 7.20 |
| HPO42− ⇌ PO43− + H+ | 12.37 |
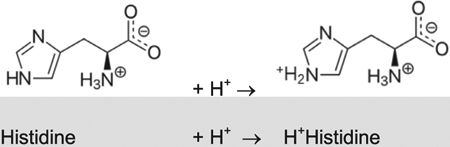
| 6.0–6.5 | |
 | |
