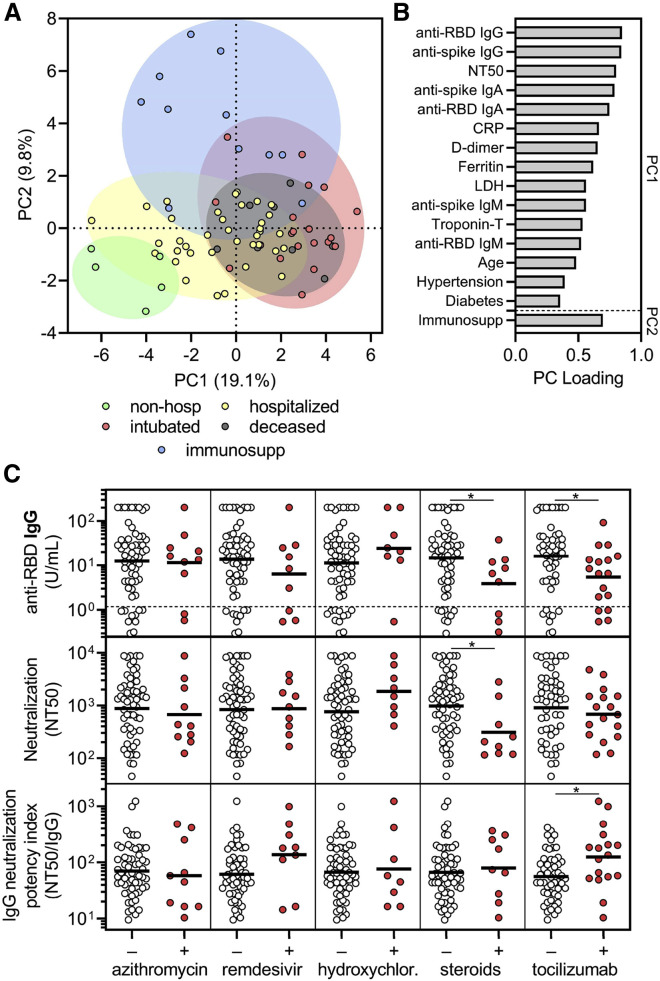
Figure 5.
Corticosteroid and tocilizumab therapy decrease humoral immune responses to SARS-CoV-2
(A and B) Principal components analysis was performed using the following variables: age, sex language, pre-existing medical conditions, treatments received, clinical laboratory data (ferritin, CRP, D-dimer, LDH, troponin-T, and lymphocyte nadir), anti-RBD and anti-spike antibody levels, and neutralization titers. The severity cohort of each patient is indicated by color. Patients with missing data were excluded. Loading of principle components (PC) is shown in (B).
(C) Sub-analyses of anti-RBD IgG levels (upper panel), neutralization titer (middle panel), and neutralization potency index (NT50/IgG) (lower panel) were performed on COVID-19 patients that were in the hospital for at least 3 days to (n = 69) and were performed on the last collected specimen to show the effect of azithromycin (n = 10 out of 69 received), remdesivir (n = 9 out of 69), hydroxychloroquine (n = 8 out of 69), corticosteroids (n = 9 out of 69), and tocilizumab (n = 17 out of 69; treated as part of a trial with 2:1 randomization to placebo). Several patients received more than one treatment regimen and thus were included in more than one treatment category. A t test was performed for each comparison of patients who received (+) versus did not receive (–) the indicated treatment; ∗ indicates unadjusted p < 0.05.
See also Figure S4.