Figure 1.
Selective Loss of Neuronal Autophagy Facilitates Excitatory Neurotransmission
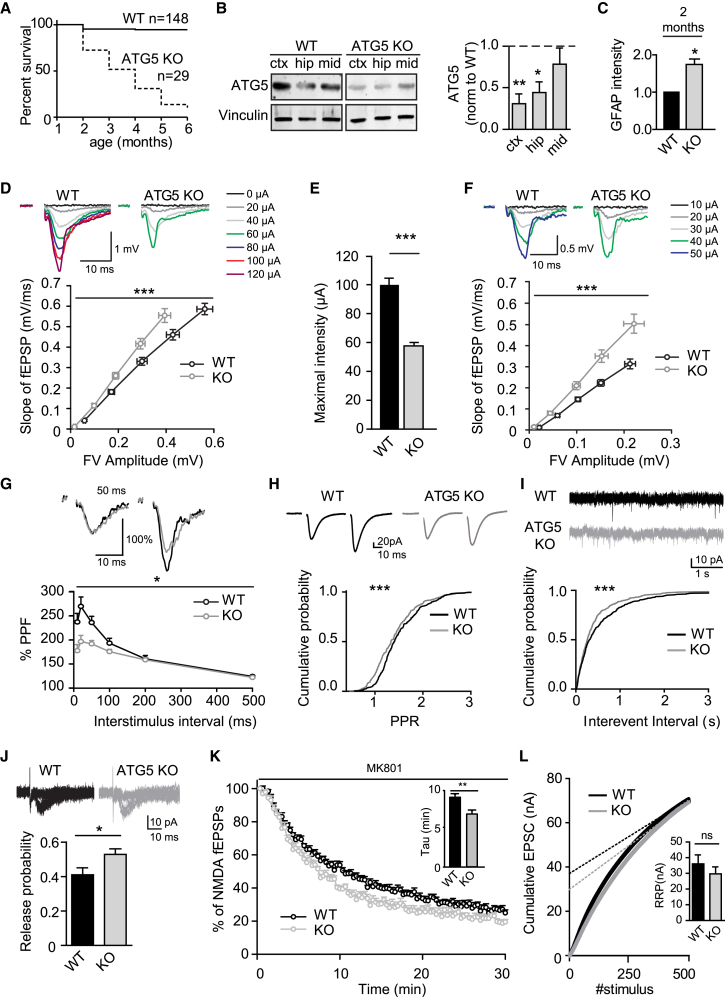
(A) Decreased survival of KO mice conditionally deleted for ATG5 by transgenic expression of Cre recombinase under the telencephalon-specific EMX promoter (ATG5flox/flox; EMX1-Cre).
(B) Western blot and quantification showing an ATG5 decrease primarily in the cortex (ctx) and hippocampus (hip) of 2-month-old ATG5-cKO mice. n = 4 mouse pairs for ctx and midbrain (mid) and n = 3 mouse pairs for hip, one-sample t test.
(C) Quantification of GFAP immunostaining in 6- to 7-week-old control and ATG5-cKO brain slices. Slices were taken from 3 mice; one-sample t test. See also Figure S1C.
(D) Basal excitatory neurotransmission measured as the relationship between fiber volley (FV) amplitudes and slopes of fEPSPs in WT control (n = 24 slices, 12 mice) and ATG5-cKO (n = 24 slices, 12 mice) mice. Representative fEPSP traces (above) and quantified data are shown. Significant difference between WT control and ATG5-cKO slices encompassing the curve; two-way repeated-measures ANOVA.
(E) A lower stimulation intensity is required to elicit maximal responses in ATG5-cKO (58.8 ± 2.1 μA) compared with control mice (100.8 ± 4.6 μA); t test.
(F) Basal excitatory neurotransmission measured as relationships between FV amplitudes and slopes of fEPSPs in WT control (n = 11 slices, 6 mice) and ATG5-cKO (n = 10 slices, 6 mice) mice in the presence of the GABAA receptor antagonist picrotoxin (50 μM). Representative fEPSP traces (above) and quantified data are shown. Significant difference between WT control and ATG5-cKO slices encompassing the curve; two-way repeated-measures ANOVA.
(G) Measurements of paired-pulse facilitation (PPF) in the presence of the GABAA receptor antagonist picrotoxin (50 μM) reveal significantly reduced PPF in ATG5-cKO (n = 10 slices, 6 mice) compared with control (n = 11 slices, 6 mice) mice. Representative traces of PPF at a 50-ms interstimulus interval (above) and quantified data over a range of interstimulus intervals (10–500 ms), given as a percentage of the second in relation to the first response (percent PPF), show reduced facilitation of the second response in ATG5-cKO mice; two-way repeated-measures ANOVA.
(H) Cumulative probability shows a left-shifted distribution for PPR in ATG5-cKO mice. n = 28 (WT) or 30 (KO) slices from 8 animals; Kolmogorov-Smirnov test.
(I) Cumulative probability distribution shows decreased interevent intervals for sEPSCs in ATG5-cKO mice. n = 17 (WT) or 21 (KO) cells from 7 and 6 animals, respectively; Kolmogorov-Smirnov test.
(J) Release probability evaluated by a minimal stimulation protocol shows increased release probability (i.e., decreased failure rate) in ATG5-cKO mice. n = 23 (WT) or 24 (KO) cells from 5 animals; t test.
(K) Release probability evaluated by NMDA receptor-mediated fEPSP amplitude decay. Averaged NMDA receptor-mediated amplitudes in the presence of MK801 (30 μM) show significantly faster decay in KO mice (see tau values in the bar graph). n = 12 (WT) or 10 (KO) slices from 7 and 6 animals, respectively; t test. See also Figures S2B and S2C.
(L) Estimation of RRP size by back-extrapolation (last 50 data points) of the cumulative EPSC to the y axis. n = 13 (WT) or 15 (KO) cells from 4 animals; Mann-Whitney test.
All data show mean ± SEM. ns, not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.