Figure 2.
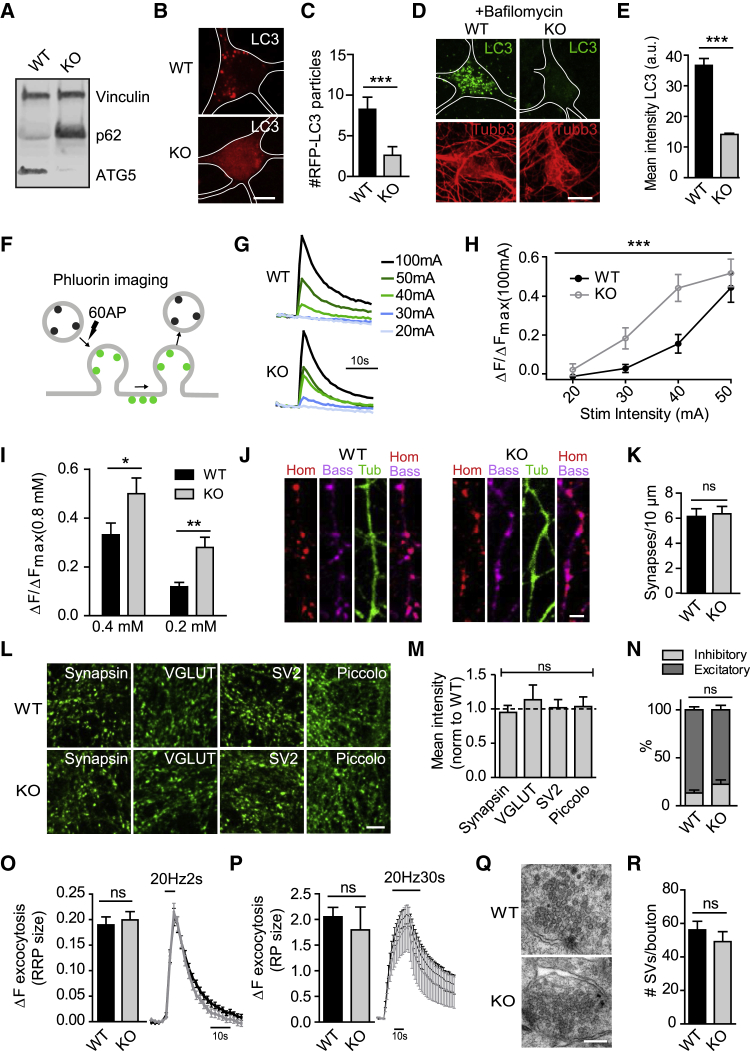
ATG5-iKO Hippocampal Neurons Display Increased Stimulation-Dependent SV Release
(A) Immunoblot showing ATG5 decrease and p62 increase in lysates from tamoxifen-inducible ATG5-iKO (KO) hippocampal cultures.
(B and C) Representative images (B) of hippocampal WT and KO hippocampal neurons expressing mRFP-LC3. Note the decreased LC3 punctum numbers in ATG5 KO neurons; quantified in (C). Scale bar, 10 μm. n = 20 cells from a representative experiment; Mann-Whitney test.
(D and E) ATG5 KO hippocampal neurons show deficient LC3-positive punctum formation upon bafilomycin treatment (10 nm, 4 h). Representative immunofluorescence images show LC3 staining in (D) (quantified in E). Scale bar, 10 μm. n = 42 cells, 1 experiment; Mann-Whitney test.
(F–H) Detection of exocytosis using Synaptophysin-pHluorin.
(F) Schematic showing reporter de-acidification during vesicle fusion with the plasma membrane.
(G) Example traces (averaged from a representative experiment) showing a stimulus-dependent decrease in pHluorin signal in WT and KO hippocampal synapses.
(H) Graph showing mean peak fluorescence upon different stimulation intensities. Values per cell are normalized to the corresponding maximal fluorescent peak at 100 mA (Fmax). n = 17–35 cells, 20 boutons per cell, 5 independent experiments; two-way ANOVA.
(I) Graph showing mean peak fluorescence of the pHluorin signal under conditions of different extracellular calcium concentrations. Values per cell are normalized to the corresponding Fmax at 0.8 mM calcium. n = 21 cells, 20 boutons per cell, 3 independent experiments; t test.
(J) Representative confocal images of hippocampal neurons immunostained for β3-tubulin (green), Homer 1 (postsynaptic, red), and Bassoon (presynaptic, magenta). Scale bar, 2 μm.
(K) Synapse numbers in WT and KO cultures expressed as the number of Homer 1/Bassoon-positive puncta along β3-tubulin-positive neurite length. n = 3 independent experiments, ~2,900 synapses per genotype; paired t test.
(L) Representative confocal images of hippocampal neurons immunostained for Synapsin-1, VGLUT1, SV2, and Piccolo. Scale bar, 5 μm.
(M) Quantification of Synapsin-1, VGLUT1, SV2, and Piccolo immunostaining intensities. The mean values for the control are set to 1, and the mean value for the KO is expressed relative to this. n = 3 independent experiments, 26-37 images per condition; one-sample t test.
(N) Percentage of inhibitory and excitatory synapses in WT and KO hippocampal cultures determined by Synapsin (marker for all synapses) and vGAT (inhibitory synapse marker) antibody staining. Excitatory synapses are Synapsin positive and vGAT negative. n = 3 independent experiments, 45–47 images per condition; paired t test.
(O) Quantification and average traces of Synaptophysin-pHluorin-expressing neurons stimulated with 40 APs (20 Hz) to determine the size of the readily releasable SV pool (RRP). n = 3 independent experiments, 20 cells per condition; paired t test.
(P) Quantification and average traces of Synaptophysin-pHluorin-expressing neurons stimulated with 600 APs (20 Hz) to determine the size of the recycling SV pool (RP). n = 3 independent experiments, 20–24 cells per condition; paired t test.
(Q and R) Representative electron micrographs of nerve terminals in WT and KO hippocampal cultures show no difference in the number of SVs per bouton (quantified in R). Scale bar, 1 μm. n = 41 (WT) and 45 (KO) boutons, 1 experiment; Mann-Whitney test.
All data represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.