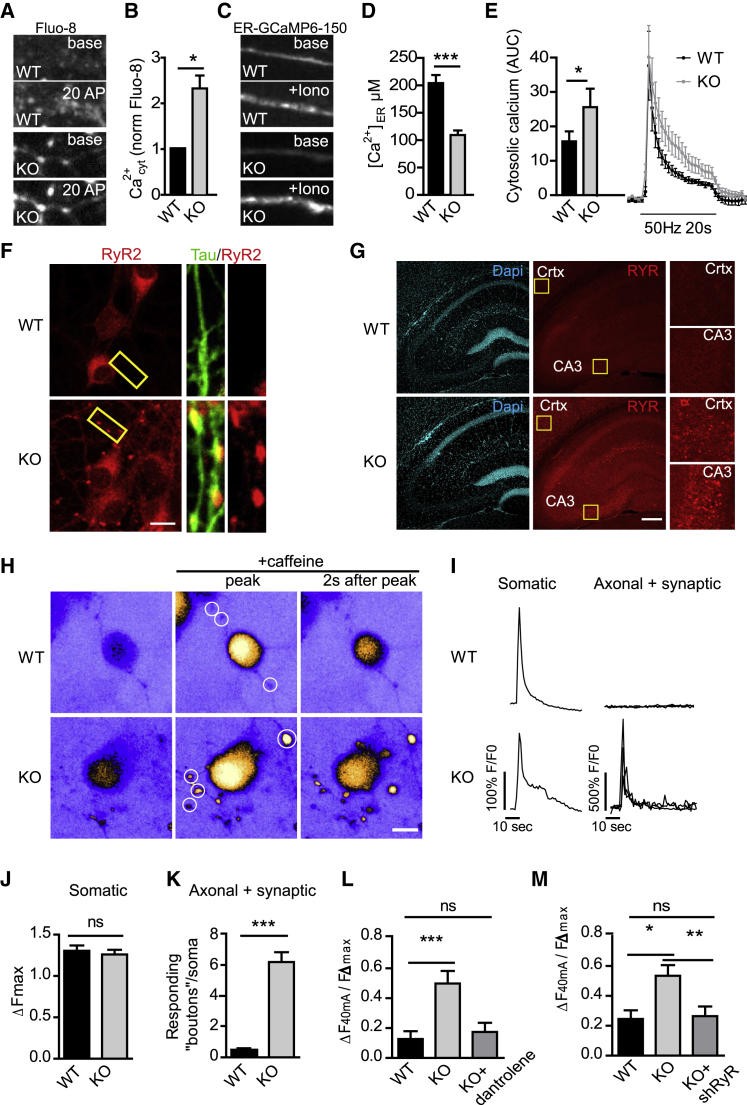
Figure 6.
Increased RyR-Mediated Calcium Release Underlies Elevated Neurotransmission in ATG5 KO Neurons
(A–E) Impaired calcium homeostasis in ATG5-iKO hippocampal neurons.
(A and B) Hippocampal neuron cultures were probed with the fluorescent Ca2+-binding dye Fluo-8 to measure cytosolic calcium in neurites. Neurites were identified by a mild electrical stimulation (20 APs) causing a Fluo-8 increase. Fluorescence intensities of baseline Fluo-8 (before stimulus) are quantified in (B). Calcium levels in WT neurons were set to 1. n = 4 independent experiments, 36 images for WT and 38 images for KO; one-sample t test.
(C) Hippocampal neuron cultures were transfected with ER-GCaMP6-150, and axons were imaged before and after 50 μM ionomycin application to induce indicator saturation for calibration.
(D) Average peak fold change in fluorescence during ionomycin application is used to estimate resting ER calcium concentration in the axon. n = 30 axons, 3 independent experiments; unpaired t test.
(E) Calcium buffering in the presynapse was measured by infecting neurons with the synaptophysin-GCaMP6 virus. The fluorescence change in response to a 50-Hz, 20-s pulse was measured. Average traces are indicated on the right, and the area under the curve (AUC) is plotted on the left. n = 5 independent experiments, 58–63 cells per condition; paired t test.
(F) Hippocampal neuron culture immunostained for endogenous RyR2 and the axonal marker Tau. Scale bar, 5 μm.
(G) Images of mouse brain sections showing an increase in RyR immunoreactivity in ATG5-cKO crtx and the hippocampal CA3 area. Yellow boxes indicate magnifications shown on the right. See also Figure S6A for quantifications. Scale bar, 200 μm.
(H–K) Increased caffeine-induced calcium release from the ER in ATG5-iKO hippocampal neurons in culture.
(H) Heatmap images showing Fluo-8 calcium responses during a 20-mM caffeine pulse. Scale bar, 10 μm.
(I) Representative traces from Fluo-8 responses in the somata or axonal areas (indicated by white circles in H).
(J) Maximum Fluo-8 intensity increase in WT and ATG5-iKO somata. n = 100 WT and 98 KO cells from two independent experiments; unpaired t test.
(K) Average number of responding “boutons” per soma. n = 90 WT and 95 KO somata from two independent experiments; Mann-Whitney test.
(L) Detection of exocytosis using Synaptophysin-pHluorin in WT and ATG5-iKO hippocampal neurons. A graph shows mean normalized peak fluorescence upon 40-mA stimulation. Dantrolene (10 μM), a RyR inhibitor, rescues increased responses in ATG5-iKO neurons. Values per cell are normalized to the corresponding maximal fluorescent peak at 100 mA (Fmax). n = 18–22 cells, 3 independent experiments; one-way ANOVA with Tukey’s post-test.
(M) Ryanodine receptor (RyR) knockdown decreases exocytosis in ATG5-iKO neurons. Values per cell are normalized to the corresponding Fmax at 100 mA. n = 13 WT or 25–27 KO cells, 4 independent experiments; one-way ANOVA with Tukey’s post-test.
All data represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.