Fig. 3.
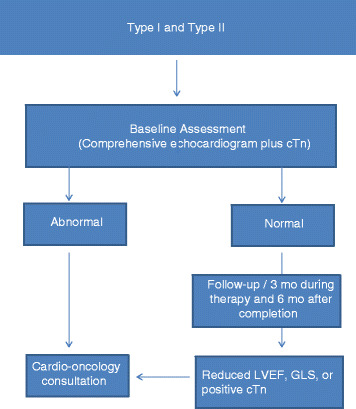
Type I and Type II cardiotoxicity. Baseline and serial evaluation in patients receiving combined therapies with drugs with both type I and type II toxicity risk. Echocardiogram and cardiac biomarkers are performed during baseline. For abnormal baseline screening, we suggest cardio-oncology consultation. For normal baseline screening, we suggest serial monitoring with echocardiogram and biomarkers every 3 months during therapy and 6 months after completion of treatment. F/U indicates follow-up; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; cTn, serum cardiac troponin
