Abstract
Background and objectives
It is essential to know the proportion of health care workers (HCW) who are COVID 19 positive, as well as the severity and mortality among them.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic review and meta-analysis. Databases including PubMed, EMBASE and Web of Science were searched from December-31, 2019 to April-23, 2020. The search was limited to the studies that reported the data on the number of COVID-19 positive healthcare workers, among the COVID-19 positive patients. Case reports, duplicate publications, reviews, and family-based studies were excluded. The methodological quality of studies was assessed by the Appraisal tool for Cross-Sectional Studies (AXIS) tool.
Results
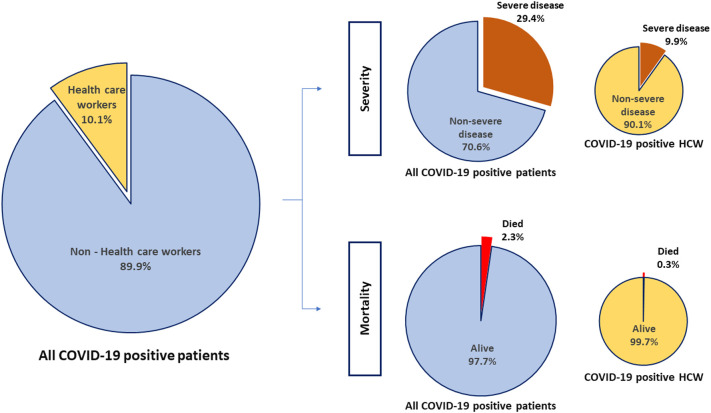
In this systematic review and meta-analysis, we pooled eleven studies to investigate the above factors. The overall proportion of HCW who were SARS-CoV-2 positive among all COVID-19 patients was 10.1% (95%CI: 5.3–14.9). This proportion varied according to the country of study i.e. China (7 studies) - 4.2%, 95%CI:2.4–6.0; United States (3 studies) – 17.8%, 95%CI:7.5–28.0; and Italy (1 study) – 9.0%, 95%CI:8.6–9.4. The incidence of severe or critical disease in HCW (9.9%, 95%CI:0.8–18.9) was significantly lower (p < 0.001) than the incidence of severe or critical disease in all COVID-19 positive patients (29.4%, 95%CI:18.6–40.2). Similarly, the mortality among HCW (0.3%, 95%CI:0.2–0.4) was also significantly lower (p < 0.001) as compared to that of all patients (2.3%, 95%CI:2.2–2.4).
Conclusion
Health care workers who are COVID-19 positive constituted a significant proportion of all COVID-19 patients; but the severity and mortality were lower among them.
Highlights
-
•
Meta-analysis of eleven studies showed that nearly 10% of COVID-19 positive patients are health care workers
-
•
The incidence of severe disease in health care workers (9.9%) was significantly lower than its incidence among all COVID-19 positive patients (29.4%)
-
•
The mortality among health care workers (0.3%) was also significantly lower as compared to that of all patients (2.3%)
1. Introduction
The outbreak of novel coronavirus disease 2019 (COVID-19) started in Wuhan, Hubei Province, China in late December 2019. The resulting pandemic has infected 5.7 million people globally as of May 29, 2020 [1]. The recent spread of COVID-19 globally led to considerable anxiety and concern among health care workers (HCW) [2]. HCWs are at risk for infection caring for infected patients. They understandably worry not only about becoming infected but also infecting co-workers, patients and family members. In the SARS and MERS outbreak health care workers made up around a quarter of those infected [3,4].
The primary objective of this systematic review was to determine the proportion of HCW who were COVID-19 positive among all the COVID-19 patients. Secondary objectives were to find out the incidence of severe or critical disease and deaths among HCW. Comparison of incidence in HCW and all COVID-19 patients were also investigated.
2. Materials and methods
2.1. Data sources and searches
This systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA guidelines) [5]. Databases including PubMed, EMBASE and Web of Science were searched from December-31, 2019 to April-23, 2020. Two independent investigators (AK, SB) searched the databases using search terms like ‘2019-ncov’, ‘novel coronavirus 2019’, ‘COVID-19’, ‘SARS-CoV-2’, ‘Wuhan coronavirus’, ‘health care worker’, ‘health care professional’, ‘physician’ and ‘medical staff’ (search query available in Supplement Table S1). There were no restrictions in terms of country, publication language or publication date. Reference lists of all relevant articles and ‘related citation’ search tool of PubMed were checked for any additional publications.
2.2. Selection criteria
Study selection was performed by two independent investigators (AK, SB). We included studies that reported the data on the number of COVID-19 positive HCW, among the COVID-19 positive patients. Case reports, duplicate publications, reviews, and family-based studies were excluded. Discrepancies between reviewers were resolved in the presence of a third reviewer (RM).
2.3. Data abstraction and quality assessment
Data collected included study characteristics such as authors, publication date, study design, country, study sample size (i.e. the number of COVID-19 positive patients), number of COVID-19 infected HCW, the number of severe/critical disease in HCW and all patients, definition used for severe/critical disease in the study, and the number of deaths in HCW and all patients. One reviewer (AV) extracted the data and second reviewer verified the data independently (SB). The methodological quality of the studies were assessed with the Appraisal tool for Cross-Sectional Studies (AXIS) tool [6]. Two authors (AV, PA) performed the quality assessment separately and disagreements were resolved by consensus in the presence of a third reviewer (JN). In AXIS tool, for every correct answer, the score of one was assigned to each of the twenty questions.
2.4. Quantitative data synthesis
The total number of healthcare workers infected with COVID-19 (numerator) and the total number of COVID-19 positive patients (denominator) were extracted for calculation of proportion, and these proportions were summarised by using random-effects meta-analysis. To assess the heterogeneity among studies, inconsistency statistics (I2) were calculated. Significant heterogeneity was considered to be present when I2 was >50% [7]. Traditional funnel plots were found to be inaccurate in the assessment of publication bias in meta-analysis of proportions [8]. Hence, funnel plots were not prepared. The p-value for Egger's regression coefficient <0.10 was considered as significant publication bias [9]. Subgroup analysis, according to the country of publication, was undertaken. To compare the incidence of severe/critical disease and deaths in HCW versus all patients, the chi-square test was utilized (p < 0.05 was considered statistically significant) [10,11]. All data were collected in Microsoft Excel Spreadsheet (Office 365). Random-effects analysis, generation of forest plot, assessment of heterogeneity and publication bias were performed with the METAPROP platform for STATA (version-14.2); StataCorp, College Station, TX).
3. Results
3.1. Search results and study characteristics
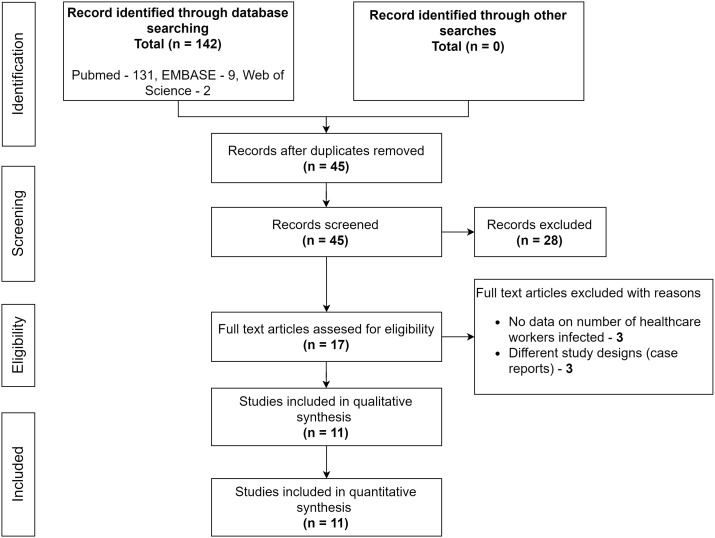
The literature search flow diagram is summarised in PRISMA format (Fig. 1) and detailed PRISMA checklist is available in Supplement Table S2. Using our search criteria, we identified 142 studies, of which 131 were from PubMed, nine were from EMBASE, and two were from Web of Science. A total of 45 records were screened after removal of duplicates. A total of 17 full-text articles were assessed for eligibility, and six articles were excluded due to various reasons, as shown in Fig. 1. Finally, eleven studies were included in this meta-analysis [3,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
3.2. Characteristics of the included studies
A total of eleven studies, consisting of 119,216 patients (including 13,199 HCW), were selected for this meta-analysis (Table 1). Studies were published recently between January 29, 2019 to April 21, 2020. Individual study population size ranged between 9 and 49,370 laboratory-confirmed COVID-19 patients. Among the included studies, seven were from China [3,[12], [13], [14], [15], [16], [17]], three were from the United States [[19], [20], [21]] and one from Italy [18]. All eleven studies provided information on the number of HCW infected, as well as the total number of COVID-19 patients. Incidence of severe and critically ill among HCW was reported in 4 studies, i.e. by Feng et al. [15], Wang et al. [14], Burrer et al. [20] and Goyal et al. [21]. Incidence of severe and critically ill among all COVID-19 patients was reported in 4 studies, i.e. by Feng et al. [15], Wang et al. [14], Zhang et al. [16] and Goyal et al. [21]. There were variations in the definition of severe/critical disease across the included studies. Feng et al. [15] and Zhang et al. [16] defined ‘severe’ disease as presence of dyspnea, tachypnea (RR ≥ 30/min), oxygen saturation (≤ 93%), PaO2/FiO2 ratio < 300, and/or > 50% lung infiltrates; and ‘critical’ disease as multiorgan dysfunction and/or ICU admission. Wang et al. [14] and Burrer et al. [20] defined ‘severe/critical’ disease as ICU admission only, whereas Goyal et al. [21] included patients requiring invasive mechanical ventilation as ‘severe/critical’ disease. Similarly, separate COVID-19 specific mortality data of HCW were reported in 2 studies (Feng et al. [15] and Burrer et al. [20]) and of all patients were reported in 2 studies (Feng et al. [15] and McMichael et al. [19]). Results of the quality assessment of the included studies are summarised as AXIS scores in Table 1 and the detailed risk of bias analysis is available in Supplement Table S3. The overall quality score of studies was sixteen out of twenty. Six studies had a quality score of eighteen [12,14,15,17,19,21], one study of sixteen [16], two studies of fifteen [3,20] and one study of twelve [13]. Quality of the study by Livingston et al. [18] could not be assessed as detailed data could not be retrieved.
Table 1.
Characteristics of the included studies in the systematic review and meta-analysis.
| Author [reference] | Country | Publication date | Number of HCW infected (a) | Number of total COVID-19 patients (b) | Proportion {(a/b)a 100%} | Severe and critical disease among HCW, n (%) | Severe and critical among disease all, n (%) | Death among HCW, n (%) | Overall deaths, n (% overall) | Quality of study (score out of 20)a |
|---|---|---|---|---|---|---|---|---|---|---|
| Li [12] | China | January 29, 2020 | 15 | 370 | 4.05 | – | – | – | – | 18 |
| Mingquiang Z [13] | China | February 3, 2020 | 1 | 9 | 11.11 | – | – | – | – | 12 |
| Wang [14] | China | February 7, 2020 | 40 | 138 | 28.99 | 1 (2.5) | 36 (26.1) | – | – | 18 |
| Feng Z (China CDC) [15] | China | February 17, 2020 | 1716 | 44672 | 3.84 | 247 (14.39) | 8255 (18.48) | 5 (0.3) | 1023 (2.29) | 18 |
| Zhang [16] | China | February 18, 2020 | 3 | 140 | 2.14 | 0 (0) | 58 (41.43) | – | – | 16 |
| Guan [17] | China | February 28, 2020 | 38 | 1099 | 3.46 | – | – | – | – | 18 |
| Xuequi L [3] | China | March 11, 2020 | 4 | 346 | 1.16 | – | – | – | – | 15 |
| Livingston [18] | Italy | March 17, 2020 | 2026 | 22512 | 9.00 | – | – | – | – | couldn't be done |
| McMichael T [19] | United States | March 27, 2020 | 50 | 167 | 29.94 | – | – | 0 (0) | 35 (20.96) | 18 |
| Burrer S (USA CDC) [20] | United States | April 14, 2020 | 9282 | 49370 | 18.8 | 184 (0.02) | – | 27 (0.003) | – | 15 |
| Goyal [21] | United States | April 21, 2020 | 24 | 393 | 6.11 | 8 (33.33) | 130 (33.08) | – | – | 18 |
CDC – Center for Disease Control and Prevention, HCW – healthcare workers, Severe disease – dyspnoea, tachypnoea (respiratory rate > 30/min), critical disease – respiratory failure, septic shock or multiorgan dysfunction.
Scores for each study in Appraisal tool for Cross-Sectional Studies (AXIS) tool.
3.3. Quantitative data synthesis results
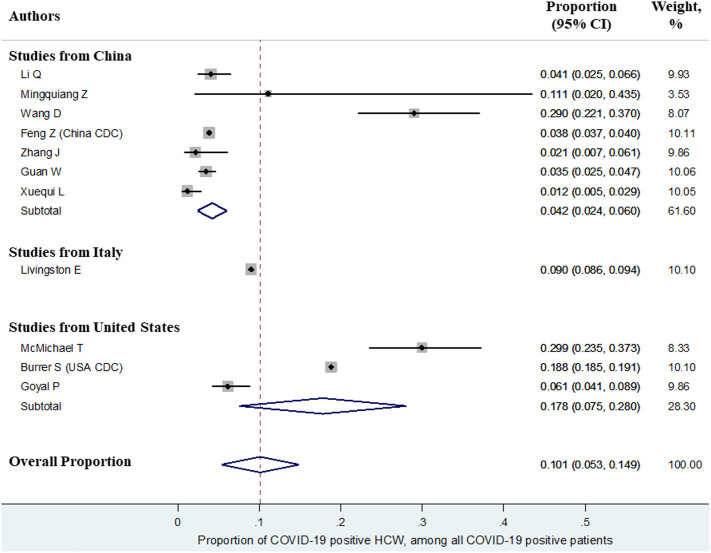
The overall proportion of HCW (n = 13,199) who were SARS-CoV-2 positive among all COVID-19 patients (n = 1,19,216) was 10.1% (95%CI:5.3–14.9). This proportion varied according to the country of study i.e. China (7 studies) - 4.2%, 95%CI:2.4–6.0; United States (3 studies) – 17.8%, 95%CI:7.5–28.0; and Italy (1 study) – 9.0%, 95%CI:8.6–9.4. Forest plot summarizing the proportions as well as subgroup analysis by country is depicted in Fig. 2 .
Fig. 2.
Forest plot showing the overall proportion of health care workers infected with COVID-19 among all patients of COVID-19.
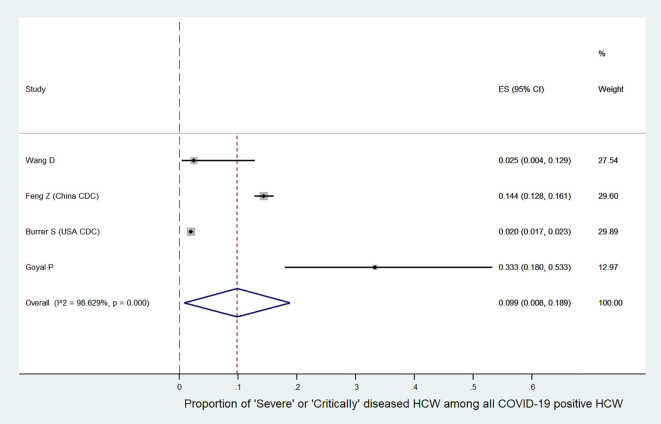
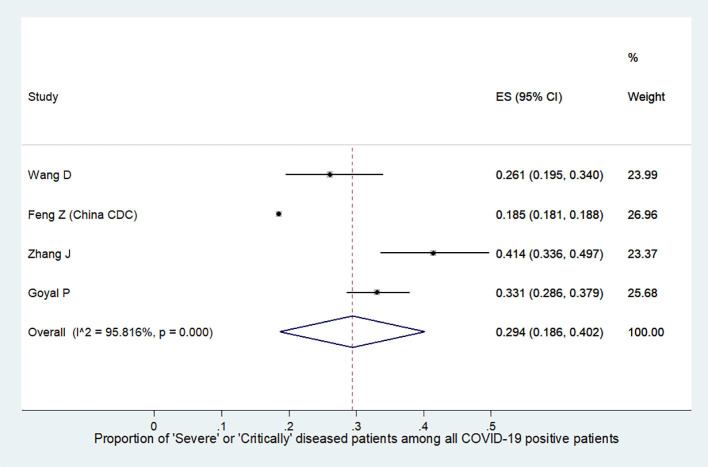
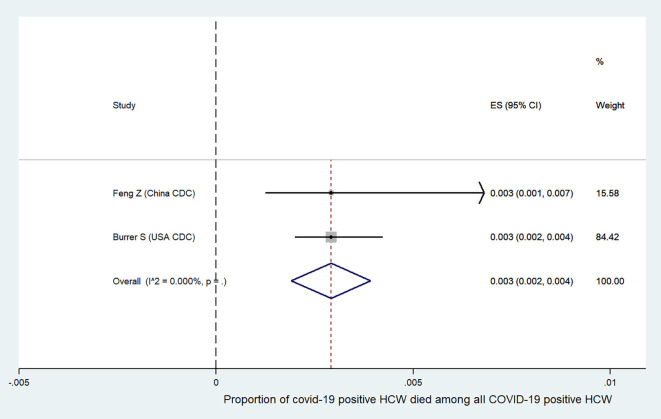
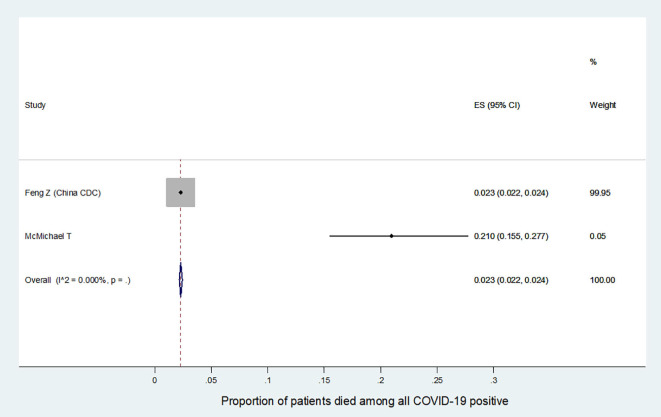
We further analyzed the available information to find out the severity of COVID-19 among the affected HCW. It was found that the incidence of severe or critical disease in HCW (9.9%, 95%CI:0.8–18.9, Fig. S1) was significantly lower than the incidence in all COVID-19 patients (29.4%, 95%CI:18.6–40.2, Fig. S2) {difference:19.5%, 95%CI:18.79–20.19, p < 0.001}. The mortality among HCW (0.3%, 95%CI:0.2–0.4, Fig. S3) was also significantly lower as compared to the mortality in all COVID-19 patients (2.3%, 95%CI:2.2–2.4, Fig. S4) {difference:2%, 95%CI:1.82–2.17, p < 0.001}. Summary of all the above results are depicted in an infographic form, in Fig. 3.
Fig. S1.
Summary proportion of ‘severe or critically diseased’ HCW among all COVID-19 positive HCW.
Fig. S2.
Summary proportion of ‘severe or critically diseased’ patients among all COVID-19 positive patients.
Fig. S3.
Summary proportion of COVID-19 positive HCW ‘died’ among all COVID-19 positive HCW.
Fig. S4.
Summary proportion of COVID-19 positive patients ‘died’ among all COVID-19 positive patients.
Fig. 3.
Infographic showing the overall proportion of health care workers (HCW) who were COVID-19 positive among all COVID-19 patients, and the proportion of severe disease and mortality in HCW and all patients.
Among the included studies, significant statistical heterogeneity was observed (I2–99.8%). Subgroup analysis according to the country showed that studies from China had a significant statistical heterogeneity (I2–90.9%), as well as from the US (I2–98.3%). Egger's regression demonstrated the absence of evidence of publication bias (p – 0.61).
4. Discussion
Our systematic review and meta-analysis showed that the overall proportion of HCW who were SARS-CoV-2 positive among all COVID-19 patients was nearly 10%. This was in contrast to the percentage of HCW infected during SARS epidemic, which was much higher in the range of 21 to 26.31% [3,4]. The subgroup analysis showed a significant variation in the proportion that was observed country-wise, ranging from 4.2% in Chinese studies to 17.8% in American studies. This might be explained due to the lack of adequate preparation in the West to meet the surge of patients, which led to the acute shortage in the personal protective equipment [22,23].
More than half of the COVID-19 positive HCW reported that they had contact with COVID-19 positive patients in healthcare settings. However, there were also known exposures in households and community settings [20]. Hence, HCW should take the utmost care during their duties in hospitals and should prioritize precautions like hand hygiene and social distancing in the community
The data on HCW specialties and hospital area was investigated only in one of the included studies [14]. Data on the specialty of HCW and the area of the hospital where they were exposed were not available in most of the studies. Only Wang et al. had reported that among the affected health care workers, 77.5% worked on general wards, 17.5% in the emergency department and 5% in the ICU [14]. McMichael et al. had described the incidence of COVID-19 among HCW working in long term care facilities, and showed that the temporal and geographical transmission of the disease was in part due to the movement of HCW from one facility to another [19]. A retrospective study by Ran et al. concluded that high risk departments, long duty hours, and suboptimal hand hygiene after contact with patients were linked to COVID 19 infection. The study by Chu et al. highlighted that the atypical manifestations of COVID-19, infectivity during the incubation period, lack of sufficient protective equipment and inpatient caregivers and visitors add to the infection risk among HCW [24].
We further analyzed the incidence of severe or critical disease and deaths among the affected HCW. This was nearly three times lower compared to all positive COVID-19 patients (9.9% vs 29.4%), and the mortality among HCW was seven times lower compared to all positive COVID-19 patients (0.3% vs 2.3%). These facts might be explained by the younger age of HCW and lesser prevalence of comorbidities as compared to non-HCW [19]. This also could be attributed to the early accessibility of an HCW to the health care system and their better knowledge of the disease process. The severe disease was seen more in older HCWs, similar to the non-HCW. The study from US showed only 6% of HCWs were aged >65 years but 37% of the deaths occurred among this age group [20]. This highlights the need to protect our older HCWs, by involving them in an administrative capacity rather than direct patient care.
The high-risk exposure among HCW highlights the need for contact tracing and screening of all HCW for fever and respiratory symptoms at the shift beginning. There is also a need for considering the psychological impact on the HCW while working in a highly infected area, which can severely hamper the staff's functioning. Training all HCW on preventive measures including hand hygiene and PPE use is another promising strategy to reduce transmission in health care settings. Improving surveillance through routine reporting is necessary for monitoring the impact and effect of infection prevention and control measures.
5. Limitations
There are several limitations in our study. The studies included in our analysis were only from three countries, while >200 countries have been affected by COVID-19. Out of the eleven studies included, only four studies had described the incidence of severe and critical disease, and two of the studies had provided information on incidence of deaths in HCW. There were differences in the definition of severe or critical disease across the included studies. A significant statistical heterogeneity was observed among the included studies. Finally, it is possible that new studies were published between the completion of the literature review and when this article was completed. However, we are not aware of any new publication since that time.
6. Conclusion
There is considerable risk of contracting COVID19 infection among HCWs, but the incidence of severe disease and deaths was significantly low. This data underscores the vulnerabilities of frontline healthcare workers in tackling novel and highly transmissible pathogens. Early recognition, identification and isolation with implementation of appropriate infection prevention and control measures are imperative.
The following are the supplementary data related to this article.
PubMed search criteria.
PRISMA Checklist.
Assessment of methodological quality of the included studies were using Appraisal tool for Cross-Sectional Studies (AXIS) tool.
Author declaration
The work has not been published previously, not under consideration for publication elsewhere and is approved by all authors. If the work is accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Funding
The authors did not receive any financial support from any source.
CRediT authorship contribution statement
Ankit Kumar Sahu: Conceptualization, Methodology, Software, Data curation, Formal analysis, Writing - original draft, Supervision. V.T. Amrithanand: Writing - original draft, Visualization, Supervision, Methodology. Roshan Mathew: Conceptualization, Writing - review & editing, Visualization, Supervision, Methodology. Praveen Aggarwal: Conceptualization, Investigation, Resources, Data curation, Writing - review & editing, Supervision. Jamshed Nayer: Conceptualization, Writing - review & editing, Visualization, Supervision, Methodology. Sanjeev Bhoi: Writing - review & editing, Supervision.
Declaration of competing interest
The authors did not have any conflicts of interest.
References
- 1.World Health Organisation COVID-19 situation reports - 130. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 2.Adams J.G., Walls R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020;323:1439. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 3.Xueqiu L., Wenfeng C., Lifen H., Chun C., Yufei L., Zhoubin Z. Comparison of epidemic characteristics between SARS in2003 and COVID-19 in 2020 in Guangzhou. Chinese Journal of Epidemiology. 2020;41:634–637. doi: 10.3760/cma.j.cn112338-20200228-00209. [DOI] [PubMed] [Google Scholar]
- 4.Hsin D.H.-C., Macer D.R.J. Heroes of SARS: professional roles and ethics of health care workers. J Infect. 2004;49:210–215. doi: 10.1016/j.jinf.2004.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 6.Downes M.J., Brennan M.L., Williams H.C., Dean R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 11.Richardson J.T.E. The analysis of 2 × 2 contingency tables-yet again. Statist Med. 2011;30:890. doi: 10.1002/sim.4116. [DOI] [PubMed] [Google Scholar]
- 12.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mingqiang Z., Xiaohui W., Kaili C., Jingquan Z., Cunliang C., Yulin A. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Chinese Journal of Tuberculosis and Respiratory Diseases. 2020;43:215–218. doi: 10.3760/cma.j.issn.1001-0939.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chinese Journal of Epidemiology. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. all.14238. [DOI] [PubMed] [Google Scholar]
- 17.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 19.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020 doi: 10.1056/NEJMoa2005412. NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrer, Team CC-19 R Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. New England Journal of Medicine. 2020;0 doi: 10.1056/NEJMc2010419. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter D.J. Covid-19 and the stiff upper lip — the pandemic response in the United Kingdom. New England Journal of Medicine. 2020;0 doi: 10.1056/NEJMp2005755. null. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum L. Facing Covid-19 in Italy — ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020 doi: 10.1056/NEJMp2005492. NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 24.Chu J., Yang N., Wei Y., Yue H., Zhang F., Zhao J. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PubMed search criteria.
PRISMA Checklist.
Assessment of methodological quality of the included studies were using Appraisal tool for Cross-Sectional Studies (AXIS) tool.