OVERVIEW: THE CONCEPT OF COGNITIVE RESERVE
The aging of the population, which is accompanied by an increasing prevalence of Alzheimer disease (AD), makes it imperative to identify factors that reduce risk of onset of dementia. The increasing evidence that AD pathologic process begins to be deposited in the brain by mid-life1 has led to an expanding focus on potentially modifiable lifestyle factors that may impact an individual’s risk of cognitive decline and dementia. One such commonly studied factor is cognitive reserve (CR). The concept of CR grew out of observations that there can be a marked discrepancy between an individual’s clinical symptomatology and estimates of the amount of neuropathologic process in the brain. For example, an early study by Stern and colleagues2 that began investigating this issue reported that among individuals with probable AD, and matched for clinical severity, those with more years of education had more advanced pathologic process, as indicated by less cerebral blood flow in AD-vulnerable regions.
It has been proposed that lifetime experiences that are associated with cognitive stimulation (such as years of education, occupational attainment, and engagement in mentally stimulating leisure activities) modify the brain in a way that allows individuals to tolerate greater levels of neuropathologic process or injury before showing symptoms of functional decline.3 Although the concept of CR has primarily been studied within the context of AD, it is hypothesized to apply to any brain disease or condition that results in brain damage, and an increasing number of studies support this proposal.4–6 It has also been proposed that CR moderates the relationship between brain changes and age-related cognitive decline.3,7
The last decade has seen an increased interest in the concept of CR and related mechanisms of resilience and brain maintenance, as evidenced by several recent consensus papers and proposed research frameworks.8–10 Although there remains debate about the exact definitions of these terms, it is widely agreed that CR reflects a property of the brain that allows for sustained clinical or cognitive performance in the presence of age-related or disease-related changes in the brain.
In this review, which represents a slightly updated version of an earlier publication,11 we first briefly summarize the major lines of evidence in support of the concept of CR within the context of AD. We then provide a detailed review of longitudinal biomarker studies that have examined the relationship between measures of CR, AD pathologic process, and subsequent cognitive change or impairment among individuals who were cognitively normal when first evaluated. This includes recent studies that have been published since our initial review from 2017,11 as well as an expanded discussion on possible pathways that may link CR to cognitive and clinical outcomes. We have focused on studies of individuals with normal cognition at baseline because it is now recognized that AD pathologic process begins to develop when individuals are cognitively normal, a phase of the disease commonly referred to as preclinical AD.12 As such, these types of studies provide insight into how and to what extent CR delays the onset of the symptomatic phase of the disease, which has major public health implications; it has been estimated that interventions that delay the onset of dementia by 5 years would reduce the prevalence of dementia by 50%.13
EVIDENCE IN SUPPORT OF COGNITIVE RESERVE
Supporting the concept of CR, many large prospective epidemiologic studies of initially non-demented individuals have shown that more years of education,14 greater occupational breadth and complexity,14,15 and greater lifetime engagement in cognitively stimulating activities16 are associated with a reduced risk of dementia. The evidence regarding the relationship between measures of CR and rates of change in cognition is more mixed, with many recent studies reporting little or no association between CR and rates of cognitive decline, despite evidence that individuals with higher CR have a higher performance on cognitive tests.17 It has been suggested that the differences in findings among these studies likely reflect methodological and cohort differences and, taken together, the evidence indicates that CR primarily influences baseline levels of cognitive performance.17,18 Thus, epidemiologic studies strongly support the notion that higher levels of CR are associated with better cognitive performance, as well as a reduced risk of developing dementia later in life, whereas the impact of CR on the trajectory of cognitive decline is less clear (for a review, see Pettigrew and Soldan19). Epidemiologic research on CR, however, has generally been limited by a lack of measures of underlying AD pathologic process. As such, these types of studies cannot directly examine whether and how measures of CR affect the association between levels of neuropathologic process and cognitive performance.
Thus, studies that have incorporated biomarkers, which are considered an indirect reflection of underlying neuropathologic process, are of particular importance in clarifying the mechanisms by which CR may be protective. Most studies on CR with biomarker measures of AD pathologic process have been cross-sectional in nature. A common finding of cross-sectional studies is that, at similar levels of cognitive functioning, individuals with higher CR tend to have biomarker measures reflecting higher levels of AD pathologic process in the brain. For example, atrophy measures based on magnetic resonance imaging (MRI)20–22 and levels of amyloid and tau, derived from positron emission tomographic (PET) imaging,23,24 or measured in cerebrospinal fluid (CSF),25 tend to be more abnormal among individuals with higher CR. These findings suggest that the effects of AD pathologic process on cognition are reduced in individuals with higher reserve. Some cross-sectional studies also suggest that the effects of aging on brain structure, function, and AD pathologic process may be reduced among individuals with higher CR.26–28 An important limitation of cross-sectional studies, however, is that they cannot test whether measures of CR do in fact alter future cognitive trajectories or the risk of cognitive impairment.
For this reason, prospective longitudinal studies that collect both AD biomarkers and cognitive and clinical data are essential for testing the extent to which CR is associated with reduced age-related cognitive decline or a reduced risk of cognitive impairment in the presence of AD pathologic process. As a complimentary mechanism to CR, the same factors that have been associated with CR (such as educational and occupational attainment) may also minimize the accumulation of pathologic process, a concept that has been referred to as both brain maintenance29 and resistance.9 However, appropriately addressing the concept of brain maintenance/resistance also requires longitudinal studies.
LONGITUDINAL ALZHEIMER DISEASE BIOMARKER STUDIES OF COGNITIVE RESERVE AMONG INDIVIDUALS WITH NORMAL COGNITION WHEN FIRST EVALUATED
The number of prospective longitudinal studies that have investigated the relationship between measures of CR, AD biomarkers, and longitudinal cognitive or clinical outcomes among individuals who were cognitively normal at baseline is relatively limited (Table 1). These studies have examined 3 major themes: (1) the association between baseline measures of CR and baseline AD biomarker levels in relation to the time to progress to cognitive impairment,30–35 (2) the association between baseline measures of CR and baseline AD biomarker levels in relation to the rate of change in cognition,18,36,37 and (3) the association between baseline measures of CR and the rate of change in AD biomarkers over time.31,32,38–40
Table 1.
Longitudinal studies of the association between CR and AD biomarkers among individuals with normal cognition at baseline
| Study | Outcome Variable(s) | AD Biomarkers | CR Measures | Mean Clinical Follow-Up Time in Years (SD) | Number of Cognitively Normal Subjects at Baseline | Baseline CR Biomarker Association | CR Associated with Delayed Clinical Progression/Better Cognitive Performance Accounting for Baseline Biomarker Levels | Longitudinal CR-Biomarker Association | Relationship Between Biomarker and Clinical/Cognitive Outcome Modified by CR |
|---|---|---|---|---|---|---|---|---|---|
| Soldan et al,31 2013 | Time to onset of clinical symptom of MCI; change in AD biomarkers | CSF Aβ1-42, t-tau, p-tau | Composite score (education, NART-IQ, WAIS-R vocabulary) | 8.0 (3.4), max = 17 | 239 53 progressed |
No | Yes Delayed clinical progression |
No | Yes, for CSF t-tau and p-tau. |
| Pettigrew et al,30 2017 | Time to onset of clinical symptom of MCI | Cortical thickness in AD-vulnerable regions | Composite score (education, NART-IQ, WAIS-R vocabulary) | 11.8 (3.6) max = 20 | 232 48 progressed |
No | Yes Delayed clinical progression |
– | Yes, for those who progressed 7+ y after baseline only. |
| Roe et al,33 2011 | Time to CDR ≥0.5; change in CDR-SB, Short Blessed Test, MMSE | CSF Aβ1-42, t-tau, p-tau | Education | 3.3 (2.0) | 197 26 progressed |
– | Yes, delayed clinical progression after accounting for Aβ1-42, but not significant among those with low tau/p-tau; significant among those with high tau/p-tau | – | Yes, among those with high tau or p-tau and low education, WBV was associated with faster progression. In low tau/p-tau group, neither education nor WBV associated with progression. Similar results obtained for CDR-SB and Blessed Test, but not for MMSE. |
| Roe et al,34 2011 | Time to CDR ≥0.5 | CSF Aβ1-42, t-tau, p-tau | Education, occupational attainment (6 levels) | 3.2 (1.6) | 213 14 progressed |
– | Yes Delayed clinical progression |
– | – |
| Soldan et al,32 2015 | Time to onset of clinical symptom of MCI; change in AD biomarkers | Volumes of hippocampus, entorhinal cortex, amygdala; entorhinal cortex thickness | Composite score (education, NART-IQ, WAIS-R vocabulary) | 11.1 (3.6), max = 18 | 245 57 progressed |
No | Yes Delayed clinical progression |
No | Yes, for left entorhinal cortex volume only. |
| Udeh-Momoh et al,35 2019 | Time to progression to MCI and AD dementia | CSF Aβ1-42; CSF cortisol | Composite score (education, IQ, occupation, intracranial volume) | Median = 7, max = 10 | 91 19 progressed to MCI, 10 progressed to AD dementia |
No | No | – | Yes, among those with abnormal CSF Aβ1-42 and high cortisol, higher CR score was associated with reduced risk of progression; remained significant when other biomarkers were covaried (eg, CSF t-tau). |
| Soldan, et al,18 2017 | Change in cognitive composite Z score | Composite Z score (CSF Aβ1-42, p-tau, entorhinal cortex thickness, hippocampal volume, cortical thickness in AD-vulnerable regions) | Composite score (education, NART-IQ, WAIS-R vocabulary) | 12.1 (4.2) max = 20 | 303 with clinical/cognitive data; 170 with baseline biomarker data | No | Yes, better baseline cognitive performance and faster decline after MCI symptom onset | – | No. |
| Vemuri et al,36 2015 | Change in cognitive composite Z score | Cortical PiB-PET (dichotomous); white matter hyperintensity volume, brain infarcts on fluid-attenuated inversion recovery-MRI (dichotomous) | Education/occupation score and self-reported mid/late life cognitive activity score | 2.7 | 393 | No | Yes, better baseline cognitive performance, but no difference in slope | – | No. |
| Wolf et al,37 2019 | Change in memory composite score, ADAS-Cog, CDR-SB | CSF Aβ1-42 | Education | 2.6 (2.4), max = 10 | 276 | – | – | – | Yes, those with high education showed reduced amyloid-related cognitive decline; effects remained significant when other biomarkers were covaried (CSF t-tau, p-tau, FDG-PET, ventricular volume). |
| Suo et al,39 2012 | Change in hippocampal volume | Hippocampal volume, whole-brain volume (VBM) | LEQ | 2–3, max = 3 | 151 | Yes, midlife LEQ/occupational complexity and bilateral hippocampus, and left amygdala | – | Yes, high supervisory experiences associated with less hippocampal atrophy (N = 91) | – |
| Lo & Jagust,38 2013 | Change in AD biomarkers | CSF Aβ1-42, t-tau, p-tau, FDG-PET metabolism in 5 AD-vulnerable regions, hippocampal volume | Education (tertiles), occupation (3 levels), NART errors (tertiles) | 2–3, max = 3 | 229; 35 (CSF) 103 (FDG) 228 (HCV) | No | – | Yes, higher CR associated with less decline in CSF Aβ1-42. | – |
| Walters et al,40 2018 | Change in AD biomarkers | Cortical thickness in PCC and entorhinal cortex; PiB-PET and FDG-PET metabolism in PCC and precuneus | Intellectual activity throughout life | 3 (1), max = 3.5 | 70 | Yes, intellectual activity and FDG-PET | Yes, better baseline cognitive performance, but no difference in slope | No | – |
| Pettigrew et al,50 in press | Change in AD biomarkers | CSF Aβ1-42, t-tau, p-tau, medial temporal lobe composite Z score, cortical thickness in AD-vulnerable regions; white hyperintensity volume | Composite score (education, NART-IQ, WAIS-R vocabulary) | 2.7 (2.6), max = 8.3 | 271 (CSF) 288 (MTL) 251 (AD-vulnerable regions) 277 (WMH) |
Yes, CR composite and WMH volume | – | No | – |
Abbreviations: ADAS-Cog, alzheimer’s disease assessment scale-cognition sub-scale; CDR-SB, clinical dementia rating-sum of boxes; LEQ, lifetime experiences questionnaire; MMSE, mini-mental state examination; PCS, posterior cingulate cortex.
Cognitive Reserve, Alzheimer Disease Biomarkers and Risk of Cognitive Impairment
An important question that has been addressed by studies examining the first question—the combined effects of CR and AD biomarkers on the risk of progression to cognitive impairment—is whether CR and AD biomarkers are independent predictors of risk or whether they interact to alter future risk of progression. The presence of such an interaction is very important because it would indicate that measures of CR modify the association between the biomarker in question and risk of progression, or that the protective effects of CR on the risk of progression differ for individuals with high versus low levels of the biomarker.
Two studies30,32 addressed this question by testing whether the association between structural MRI measures of brain atrophy and the time to symptom onset of mild cognitive impairment (MCI) is modified by CR, as quantified by a composite measure of CR (ie, a composite Z score composed of years of education, and measures of vocabulary and reading ability). Soldan and colleagues32 found that the baseline volumes of 3 medial-temporal lobe structures (hippocampus, entorhinal cortex, and amygdala), and the rate of change in these structures over time, were associated with the time to progress from normal cognition to symptom onset of MCI, independently of the baseline CR composite score, which was associated with a reduced risk of progression (ie, delayed symptom onset). Only 1 structure, the left entorhinal cortex volume, interacted with CR, such that smaller baseline volumes were associated with faster time to clinical symptom onset in individuals with low CR, but not in individuals with high CR. Similar results were reported by Pettigrew and colleagues,30 who found that both CR and mean cortical thickness in “AD vulnerable regions” were independently associated with risk of progression from normal cognition to MCI within 7 years of baseline. In contrast there was an interaction between baseline CR score and cortical thickness for risk of progression more than 7 years form baseline, reflecting a stronger association between low cortical thickness and risk of symptom onset among individuals with lower CR. In addition, Pettigrew and colleagues reported that the reduction in the risk of progression associated with higher CR was greater for progression after 7 years from baseline than for progression within 7 years, suggesting that the protective effect of CR decreases as AD pathologic process levels increase. Taken together, the results from these 2 studies suggest that MRI measures of atrophy in brain regions commonly affected by AD and measures of CR have relatively independent and additive effects on the risk of progression to MCI. However, these studies also provided some evidence for interactions between CR and atrophy in some brain regions, suggesting a stronger association between atrophy and risk among individuals with lower CR than higher CR.
Four other studies addressed this same question by investigating the relationship between measures of CR and CSF measures of amyloid beta (abeta), total tau (t-tau), or phosphorylated tau (p-tau) in relationship to the risk of progression to cognitive impairment.31,33–35 For the findings regarding the relationship between CR and CSF abeta, 2 of these studies reported that CR and CSF abeta measures predicted time to progress from normal cognition to MCI, but that there was no interaction between baseline levels of CSF abeta and CR (as measured by years of education33 or a composite score31). Similarly, the third study reported that fewer years of education and lower (ie, more abnormal) CSF abeta levels were significantly associated with a faster time to onset of cognitive impairment; however, the interaction between the 2 measures was not examined.33 Taken together, these findings suggest that the protective effects of CR on the risk of progression are equivalent across the observed range of CSF abeta levels and that CR and abeta have additive and independent effects on the risk of progression. This is noteworthy because CSF abeta is widely accepted as a biomarker for amyloid plaques, 1 of the primary pathologic hallmarks of AD. Interestingly, a fourth study reported a 3-way interaction between CSF abeta, a CR composite score, and CSF cortisol levels, suggesting that the protective effects of CR in relationship to abeta may interact with other lifestyle factors, such as psychosocial stress, via its impact on the hypothalamic pituitary axis.35 This finding is consistent with previous work demonstrating that increased cortisol levels may influence the clinical expression of AD pathologic process.41 However, given the small sample size of this study (n = 17 with both abnormal abeta and high cortisol), these findings await further replication.
The findings regarding the relationship between CR and CSF p-tau and t-tau suggest that there may be an interaction between CR and degree of neuronal injury, as measured by these biomarkers. Soldan and colleagues31 found an interaction between the baseline CR composite score and both t-tau and p-tau in relationship to the time to onset of symptoms of MCI. Among participants with higher baseline levels of t-tau or p-tau, the degree to which CR modified the risk of symptom onset was less than that in participants with lower levels of t-tau and p-tau, although higher CR was still associated with a delay in symptom onset in both the low and high t-tau or p-tau groups. This suggests that, as levels of neuronal injury increase in the brain, the protective effects of CR decrease, consistent with the findings by Pettigrew and colleagues30 using MRI measures of neuronal injury. This may occur because CR is unable to compensate for increasing levels of neuronal injury, or because the neural mechanisms that underlie CR break down with increasing levels of neuronal injury. The results by Soldan and colleagues31 also indicated that CSF t-tau and p-tau levels were more strongly associated with the risk of progression among individuals with higher CR than lower CR. This was due to the fact that individuals with lower CR were at significantly increased risk of progressing (because of their low CR), even when t-tau/p-tau levels were low, and thus higher t-tau/p-tau levels were associated with less additional risk. By comparison, those with higher CR, whose overall risk of developing cognitive impairment is much lower, increased tau/p-tau levels were more predictive of progression.
The findings by Roe and colleagues33 were somewhat different, as they reported a 3-way interaction between CR (as measured by years of education), t-tau/p-tau levels, and whole brain volume in relationship to the time to cognitive impairment. Among individuals with low t-tau or p-tau levels, there was no association between years of education and risk of progression; whereas among individuals with high t-tau or p-tau levels, more education was associated with a delayed time to incident cognitive impairment, particularly among those with lower brain volumes. The 2-way interaction between t-tau/p-tau levels and education (collapsed across whole brain volume) was not reported. The absence of an association between education and risk of progression among those with low t-tau or p-tau levels may reflect the somewhat smaller sample size and smaller number of individuals who became symptomatic over the course of the study (which reduces statistical power) and the relatively short follow-up duration of 3 years (compared with 8 years in Soldan and colleagues31). In addition, years of education alone tends to be less predictive of future cognitive impairment than composite CR measures that incorporate measures of literacy or vocabulary in addition to education.30,42,43 The second study by Roe and colleagues33 reported that both years of education and baseline tau or p-tau levels were predictive of incident cognitive impairment in the same model, although their possible interaction was not examined. Overall, the results of studies that have investigated the combined effects of CR and CSF AD biomarkers in relation to the risk of progression to MCI indicate that even after accounting for levels of these biomarkers at baseline, higher CR is associated with a reduced risk of symptom onset of MCI. Although the effects of abeta and CR on the time to symptom onset appear to be independent of one another, there is some evidence that the protective effects of CR are modified by CSF t-tau and p-tau levels.
Cognitive Reserve, Alzheimer Disease Biomarker and Rate of Cognitive Decline
Only 3 longitudinal studies have examined the second question mentioned above—the rate of change in neuropsychological measures of cognition in relationship to CR and AD biomarkers among individuals with normal cognition at baseline.18,36,37 Vemuri and colleagues36 operationalized CR in 2 ways, with 1 score reflecting educational and occupational attainment and the other indexing mid- and late-life cognitive leisure activities; cognitive performance was quantified with a composite Z score composed of measures from multiple cognitive domains. The results showed that higher scores on the measure of educational and occupational attainment were associated with higher cognitive scores, independent of the amount of amyloid, as measured by Pittsburgh compound B (PiB)-PET imaging, and independent of cerebrovascular disease, as measured by white matter hyperintensities and brain infarcts on fluid-attenuated inversion recovery-MRI. Importantly, there was no interaction between the measures of CR and the PET or MRI measures, suggesting similar rates of change in cognition over the follow-up period among those with higher and lower CR scores (mean follow-up 2.7 years). Consistent with these findings, Soldan and colleagues18 also reported that, independent of AD biomarker levels, higher CR (as indexed by a composite score) was associated with better cognitive composite Z scores but did not alter the rates of cognitive change while individuals were asymptomatic (mean follow-up = 11 years). In this study, AD pathologic process was quantified by a composite score combining several major biomarker types (CSF abeta and p-tau, as well as MRI measures of the hippocampus, entorhinal cortex, and AD-vulnerable cortical regions).
Due to the long follow-up period in this latter study, and the fact that a substantial number of participants had developed cognitive impairment on follow-up (n = 66), Soldan and colleagues also examined rates of change in cognition after the onset of symptoms of MCI. In line with theoretic predictions,3 individuals with higher CR showed faster rates of cognitive decline than those with lower CR after they became symptomatic.18 In addition, the mean age of onset of symptoms of MCI was strongly associated with the baseline CR score: subjects with CR scores above the median had a mean age of symptom onset that was approximately 7 years later than for those with CR scores below the median of the group.18 It is important to note that the subjects in this study were highly educated (mean of 17 years of education), so this study may underestimate the degree to which individual differences in CR may delay the symptomatic phase of AD.
Of note, the findings regarding the association between amyloid, measures of CR, and cognitive change are not entirely consistent, with Wolf and colleagues37 reporting less abeta-related functional and memory decline over a mean of 2.6 years among individuals with higher levels of education. The reasons for this discrepancy in findings are unclear, but may be related to a number of factors, including differences in baseline biomarker levels and rates of progression to clinical impairment, as well as differences in follow-up time, selective attrition, and statistical modeling approaches (for a discussion, see Pettigrew and Soldan19). Taken together, the results from studies investigating the combined effects of CR and AD biomarkers on cognitive change and time to symptom onset suggest that, after accounting for baseline pathologic process levels, CR has little impact on cognitive trajectories before symptom onset, but it does significantly delay the onset of symptoms by several years.
Cognitive Reserve and Rate of Change in Alzheimer Disease Biomarkers
Currently, there is weak evidence for the proposal that measures of CR are directly associated with the rate of change in AD biomarkers among individuals who were cognitively normal at baseline—the third question mentioned above. This is largely because the available data are limited by relatively short follow-up periods (2–4 years of longitudinal biomarker data, on average). Lo and Jagust38 reported that, among a group of 35 cognitively normal individuals, higher scores on CR proxy variables (ie, measures of education, occupation, and reading/vocabulary) were associated with less longitudinal decline in CSF abeta, but not with change in MRI hippocampal volume or fluorodeoxyglucose (FDG)-PET metabolism. Suo and colleagues39 found that high self-reported supervisory experience in midlife (a measure assumed to reflect occupational complexity) was associated with less hippocampal atrophy over time in a sample of 91 older adults. However, self-reported general cognitive activities in early, mid, or late life did not modulate rates of brain atrophy. In 3 other studies with larger samples (N = 239–288), there was no relationship between a baseline CR composite score and rates of change in CSF abeta, t-tau, and p-tau (Pettigrew C, and colleagues, submitted for publication),31 or MRI measures of the hippocampus, amygdala, entorhinal cortex,32 or AD-vulnerable cortical regions (Pettigrew C, and colleagues, submitted for publication). Likewise, Walters and colleagues40 found that self-reported intellectual activity throughout life was unrelated to the rate of change in AD biomarkers, including MRI measures of cortical thickness, FDG-PET metabolism, and amyloid on PiB-PET. Given the long prodromal period of AD, additional studies with large samples and more longitudinal biomarker data will be needed to determine to what degree CR alters the trajectories of AD biomarkers and other aspects of brain health.
PATHWAYS LINKING COGNITIVE RESERVE TO COGNITIVE AND CLINICAL OUTCOMES
Despite the strong evidence that proxy measures of CR are associated with delayed clinical symptom onset, the mechanism(s) underlying these effects remain poorly understood. Fig. 1 illustrates 4 possible pathways by which CR may alter longitudinal cognitive and clinical outcomes. (1) First, CR may reduce the risk of MCI or dementia via mechanisms that are independent of the level of specific AD-related pathologic brain changes. For example, current evidence suggests that measures of CR and levels of brain amyloid independently predict the time to symptom onset.31,33 (2) Second, CR may interact with markers of pathologic process or brain health to influence future cognitive decline or risk of progression. For instance, smaller volumes or thickness in some AD-vulnerable brain regions seem to be a stronger risk factor for developing cognitive impairment among individuals with low CR than those with higher CR.30,32 Also, the protective effects of CR on clinical outcomes seem to diminish as levels of neuronal injury increase,31 suggesting that the neural mechanisms of CR become overwhelmed by pathologic process. (3) A third pathway by which CR may influence future cognitive and clinical outcomes is by delaying the onset of age-related or AD-related brain changes, or by reducing the rate of AD pathologic process accumulation. Although current evidence for this pathway is limited, future studies with longer follow-up periods will be able to investigate this pathway. For example, recent evidence suggests that midlife vascular risk factors, including obesity, high cholesterol, hypertension, and smoking are associated with late-life amyloid accumulation.44 To the extent that these midlife vascular risk factors are associated with CR proxy measures, such as educational or occupational attainment,45–47 CR may influence the accumulation of AD pathologic process indirectly via health-related behaviors in early and mid life. These health-related behaviors may also directly reduce levels of cerebrovascular disease in the brain. For example, measures of CR have been associated with reduced levels of white matter hyperintensities,48,49 which are primarily markers of small-vessel cerebrovascular disease.50 Thus, the combined amount of pathologic process in the brain (eg, AD pathologic process plus vascular pathologic process) may be altered, thereby changing the threshold by which accumulating pathologic process has an impact on cognition. (4) A fourth pathway that has been proposed is that CR alters the association between genetic factors or aging on clinical and cognitive outcomes. Older age is the greatest risk factor for AD and both amyloid and tau pathologic process increase with age. Preliminary evidence from cross-sectional studies suggests that the association between age and AD pathologic process levels26 or age-related structural brain changes27 may be attenuated among individual with higher CR.
Fig. 1.
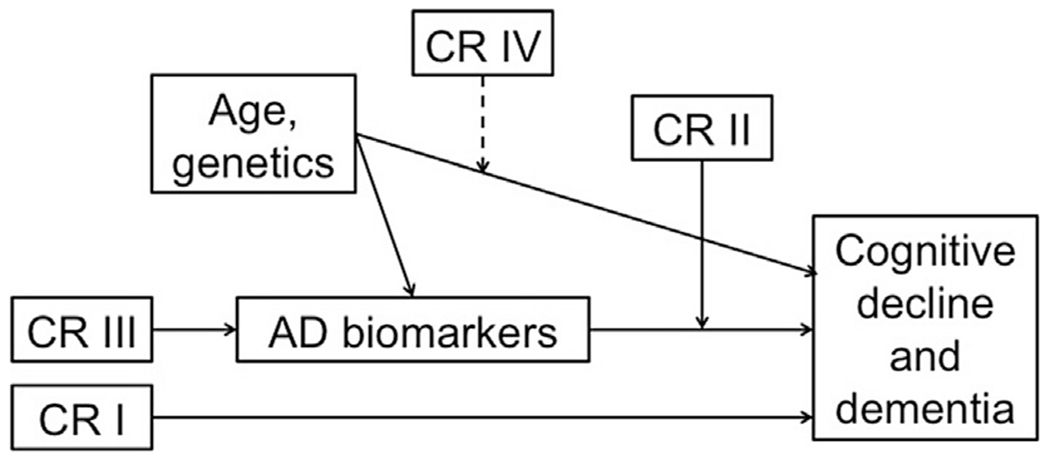
Illustration of 4 possible pathways by which cognitive reserve (CR) may influence rates of cognitive decline and risk of dementia in later life. Here, CR refers to proxy measures such as educational or occupational attainment, as well as their neural implementation(s), which are not well understood. (I) CR is linked to outcomes in a way that is, unrelated to biomarker levels. (II) CR moderates the relationship between biomarkers and outcomes. (III) CR has a direct effect on biomarker levels (ie, onset or rate of accumulation). (IV) CR modifies the relationship between age/genetics and outcomes. Dashed line indicates that although illustrated as a moderation effect, it could in fact be a mediation effect (or the relationship may depend on a specific demographic factor, gene, etc.).
An important limitation of current longitudinal studies is that they have not yet fully explored the neural mechanisms of CR. For example, neuropathological studies have suggested that resilience to AD pathologic process may be supported by maintained synaptic integrity or neural architecture.51–53 In line with this, evidence from cross-sectional suggests that CR may be implemented in the brain in the form of greater neural efficiency and speed,54–56 neural capacity, neural compensation,55,57 and greater functional connectivity.58 Longitudinal studies will be necessary to test whether these putative neuroimaging mechanisms of CR are associated with better clinical outcomes, in the same way as proxy measures of CR. If so, it might be possible to devise interventions that specifically target these neural mechanisms, thereby increasing reserve and resilience of the brain.
It has also been reported that individuals who progress from normal cognition to MCI or dementia demonstrate greater changes in CR scores over time,59 as measured by a residual approach60 (which operationalizes CR as the residual variance in cognition that is not explained by known [ie, measured] brain measures and demographic variables). This suggests that the brain mechanisms that underlie reserve may be depleted over time and greater depletions are associated with greater clinical declines. Additional studies are needed to further examine the evidence for this hypothesis.
COGNITIVE RESERVE AND PUBLIC HEALTH: PRACTICAL IMPLICATIONS
The study of CR and its neural implementation has important implications for public health. To the extent that higher CR protects against the clinical manifestations of AD by delaying the onset of the symptomatic phase of the disease, it provides an important mechanism for preserving cognitive function in old age, even while brain pathologic process levels are increasing. Current evidence suggests that higher CR is associated with approximately a 50% reduction in the risk of symptom onset of MCI30–32,43 and may delay the onset of symptoms by several years.18 As such, CR provides far greater potential benefits to individuals than any drug that is currently on the market for treating the symptoms of MCI or dementia. Moreover, by delaying the onset of the symptomatic phase of AD, CR allows older individuals to maximize daily functioning and minimize reliance on caregivers. Caring for someone with dementia is associated with enormous stress, financial strain, and negative health outcomes. Therefore, the goal of any intervention for AD should be to prolong the time that older adults are able to live independently and be active and engaged members of their family and community. Moreover, the current findings regarding CR suggest that health policies aimed at improving educational and occupational opportunities for individuals may have far-reaching consequences for future rates of cognitive decline and dementia.
KEY POINTS.
Evidence indicates that higher levels of cognitive reserve (CR) (as measured by proxy variables like educational and occupational attainment) delay the onset of symptoms of mild cognitive impairment due to Alzheimer disease (AD).
Recent findings suggest that the protective effects of CR may be independent of amyloid pathologic process, but interact with measures of neuronal injury to alter risk of cognitive impairment.
It is unclear whether CR alters future risk of cognitive decline by directly affecting brain pathologic process.
Prospective longitudinal biomarker studies are needed to investigate the mechanisms by which CR alters future risk of cognitive decline.
Acknowledgments
Funding Sources: The preparation of the article was supported in part by grants from the National Institute on Aging: U19-AG03365, P50-AG005146.
Footnotes
This article has been updated from a version previously published in Psychiatric Clinics, Volume 41, Issue 1, March 2018.
DISCLOSURE
Dr A. Soldan reports no disclosures. Dr C. Pettigrew reports no disclosures. Dr M. Albert is a consultant to Eli Lilly.
REFERENCES
- 1.Pletnikova O, Kageyama Y, Rudow G, et al. The spectrum of preclinical Alzheimer’s disease pathology and its modulation by ApoE genotype. Neurobiol Aging 2018;71:72–80. [DOI] [PubMed] [Google Scholar]
- 2.Stern Y, Alexander GE, Prohovnik I, et al. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 1992;32(3):371–5. [DOI] [PubMed] [Google Scholar]
- 3.Stern Y Cognitive reserve. Neuropsychologia 2009;47(10):2015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perneczky R, Drzezga A, Boecker H, et al. Activities of daily living, cerebral glucose metabolism, and cognitive reserve in Lewy body and Parkinson’s disease. Dement Geriatr Cogn Disord 2008;26(5):475–81. [DOI] [PubMed] [Google Scholar]
- 5.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology 2014;82(20):1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias JL, Wheaton P Contribution of brain or biological reserve and cognitive or neural reserve to outcome after TBI: a meta-analysis (prior to 2015). Neurosci Biobehav Rev 2015;55:573–93. [DOI] [PubMed] [Google Scholar]
- 7.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17(10):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement 2018. 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology 2018;90(15):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 2018;19(11):701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soldan A, Pettigrew C, Albert M. Evaluating cognitive reserve through the prism of preclinical Alzheimer disease. Psychiatr Clin North Am 2018;41(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88(9):1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271(13):1004–10. [PubMed] [Google Scholar]
- 15.Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci 2005;60(5):P251–8. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287(6):742–8. [DOI] [PubMed] [Google Scholar]
- 17.Zahodne LB, Glymour MM, Sparks C, et al. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. J Int Neuropsychol Soc 2011;17(6):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging 2017;60:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettigrew C, Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep 2019;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Julkunen V, Paajanen T, et al. Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology 2012;54(9):929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Querbes O, Aubry F, Pariente J, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain 2009;132(Pt 8):2036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sole-Padulles C, Bartres-Faz D, Junque C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2009;30(7):1114–24. [DOI] [PubMed] [Google Scholar]
- 23.Rentz DM, Mormino EC, Papp KV, et al. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the association of amyloid and tau burden on cognitive performance. Brain Imaging Behav 2017;11(2):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe CM, Mintun MA, D’Angelo G, et al. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 2008;65(11):1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumurgier J, Paquet C, Benisty S, et al. Inverse association between CSF Abeta 42 levels and years of education in mild form of Alzheimer’s disease: the cognitive reserve theory. Neurobiol Dis 2010;40(2):456–9. [DOI] [PubMed] [Google Scholar]
- 26.Almeida RP, Schultz SA, Austin BP, et al. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol 2015;72(6):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffener J, Habeck C, O’Shea D, et al. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging 2016;40:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habeck C, Razlighi Q, Gazes Y, et al. Cognitive reserve and brain maintenance: orthogonal concepts in theory and practice. Cereb Cortex 2017;27(8):3962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyberg L, Lovden M, Riklund K, et al. Memory aging and brain maintenance. Trends Cogn Sci 2012;16(5):292–305. [DOI] [PubMed] [Google Scholar]
- 30.Pettigrew C, Soldan A, Zhu Y, et al. Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain Imaging Behav 2017;11(2):357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soldan A, Pettigrew C, Li S, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol Aging 2013;34(12):2827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soldan A, Pettigrew C, Lu Y, et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp 2015;36(7):2826–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe CM, Fagan AM, Grant EA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Arch Neurol 2011;68(9):1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe CM, Fagan AM, Williams MM, et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology 2011;76(6):501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udeh-Momoh CT, Su B, Evans S, et al. Cortisol, amyloid-beta, and reserve predicts Alzheimer’s disease progression for cognitively normal older adults. J Alzheimers Dis 2019;70(2):553–62. [DOI] [PubMed] [Google Scholar]
- 36.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138(Pt 3):761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf D, Fischer FU, Fellgiebel A, et al. Impact of resilience on the association between amyloid-beta and longitudinal cognitive decline in cognitively healthy older adults. J Alzheimers Dis 2019;70(2):361–70. [DOI] [PubMed] [Google Scholar]
- 38.Lo RY, Jagust WJ. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord 2013;27(4):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suo C, Leon I, Brodaty H, et al. Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. Neuroimage 2012;63(3):1542–51. [DOI] [PubMed] [Google Scholar]
- 40.Walters MJ, Sterling J, Quinn C, et al. Associations of lifestyle and vascular risk factors with Alzheimer’s brain biomarker changes during middle age: a 3-year longitudinal study in the broader New York City area. BMJ Open 2018;8(11):e023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ennis GE, An Y, Resnick SM, et al. Long-term cortisol measures predict Alzheimer disease risk. Neurology 2017;88(4):371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manly JJ, Schupf N, Tang MX, et al. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol 2005;18(4):213–7. [DOI] [PubMed] [Google Scholar]
- 43.Pettigrew C, Soldan A, Li S, et al. Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cogn Neurosci 2013;4(3–4):136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317(14):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JP. The impact of socioeconomic status on health over the life-course. J Hum Resour 2007;42(4):739–64. [Google Scholar]
- 46.Devaux M, Sassi F, Church J, et al. Exploring the relationship between education and obesity. OECD Economic Studies 2011;2011(1). [Google Scholar]
- 47.Non AL, Gravlee CC, Mulligan CJ. Education, genetic ancestry, and blood pressure in African Americans and Whites. Am J Public Health 2012;102(8):1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wirth M, Haase CM, Villeneuve S, et al. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 2014;35(8):1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettigrew C, Soldan A, Zhu Y,et al. Cognitive reserve and rate of change in Alzheimer’s and cerebrovascular disease biomarkers among cognitively normal individuals. Neurobiology of Aging, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold SE, Louneva N, Cao K, et al. Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging 2013;34(1):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boros BD, Greathouse KM, Gentry EG, et al. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol 2017;82(4):602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Nievas BG, Stein TD, Tai HC, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 2013;136(Pt 8):2510–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speer ME, Soldan A. Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiol Aging 2015;36(3):1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steffener J, Reuben A, Rakitin BC, et al. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imaging Behav 2011;5(3):212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Habeck C, Hilton HJ, Zarahn E, et al. Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of nonverbal memory. Neuroimage 2003;20(3):1723–33. [DOI] [PubMed] [Google Scholar]
- 57.Stern Y, Zarahn E, Habeck C, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex 2008;18(4):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arenaza-Urquijo EM, Landeau B, La Joie R, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 2013;83:450–7. [DOI] [PubMed] [Google Scholar]
- 59.Bettcher BM, Gross AL, Gavett BE, et al. Dynamic change of cognitive reserve: associations with changes in brain, cognition, and diagnosis. Neurobiol Aging 2019;83:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reed BR, Mungas D, Farias ST, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 2010;133(Pt 8):2196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]


