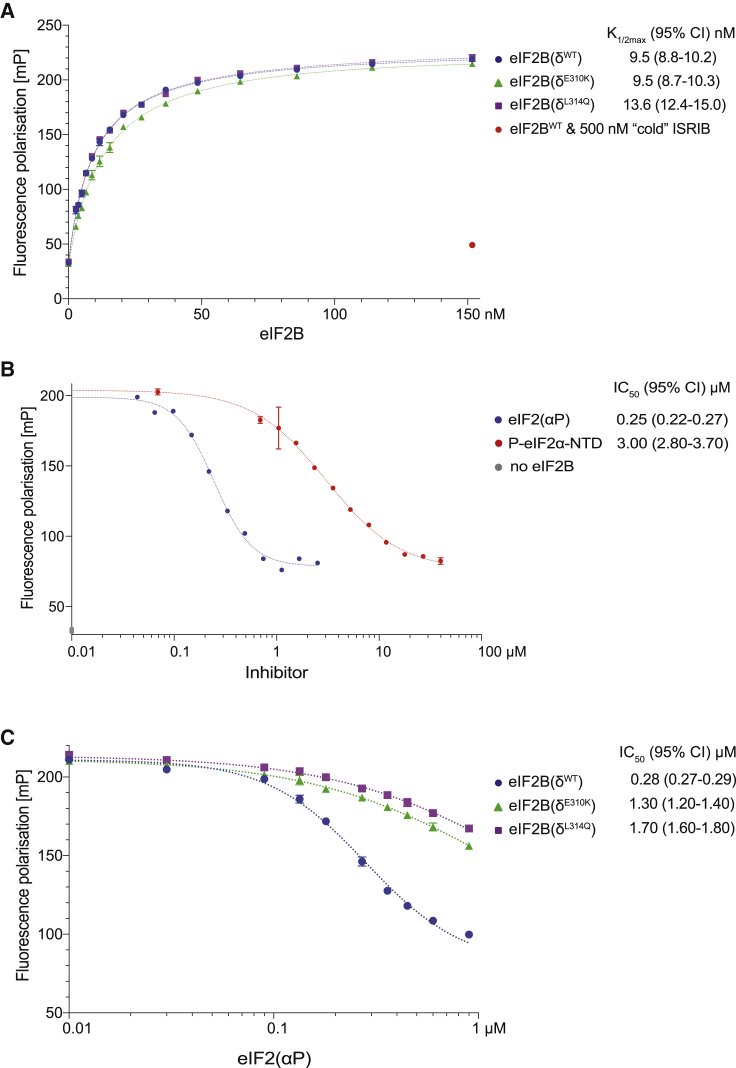
Figure 3.
Phosphorylated eIF2 Attenuates FAM-ISRIB Binding to eIF2B
(A) Plot of fluorescence polarization signals (mean ± SD, n = 3) arising from samples of FAM-ISRIB (2.5 nM) incubated with varying concentrations of wild-type or mutant eIF2B. Where indicated, 500 nM unlabeled ISRIB was added as a competitor. K1/2max with 95% confidence intervals (CI) is shown.
(B) Plot of fluorescence polarization signals, at equilibrium, arising from FAM-ISRIB bound to wild-type eIF2B in presence of the indicated concentration of the P-eIF2α-NTD (mean ± SD, n = 3) or eIF2(αP) trimer. The data were fitted by non-linear regression analysis to a “log[inhibitor] versus response four parameter” model. IC50 values with 95% CI are shown.
(C) As in (B) above, plot of fluorescence polarization signals, at equilibrium, arising from FAM-ISRIB bound to wild-type or mutant eIF2B (100 nM) in presence of the indicated concentration of eIF2(αP) trimer (mean ± SD, n = 3). IC50 values with 95% CI are shown.