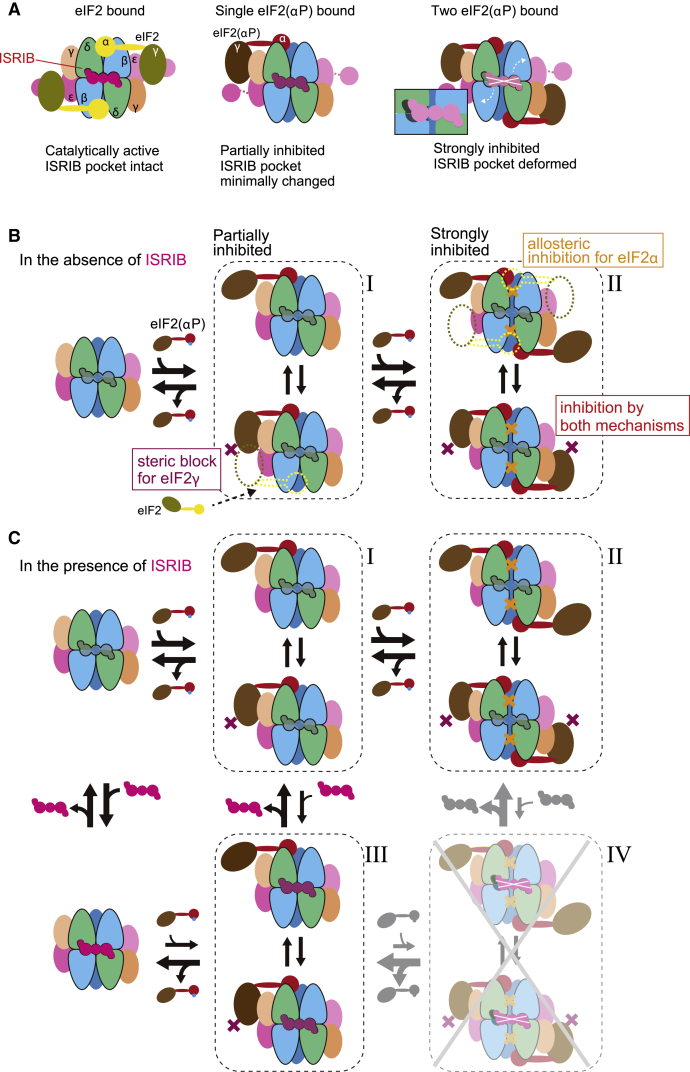
Figure 7.
A Model of the Functional Consequences of the Antagonism between eIF2(αP) and ISRIB Binding to eIF2B
(A) Cartoon of the ISRIB-binding pocket in the active (ground) state of eIF2B (left), eIF2B bound by one eIF2(αP) trimer (center), and eIF2B bound by two eIF2(αP) trimers (right).
(B) The binding of one eIF2(αP) trimer partially inhibits the catalytic activity by a steric block of the active site induced by the docking of the γ-subunit of eIF2(αP) onto eIF2Bγ (state I). Binding of a second eIF2(αP) trimer occludes the second active site of eIF2B by a similar steric block but also interferes with catalytic activity through allosteric inhibition that deforms both pockets for productive binding of eIF2α, strongly inhibiting the catalytic activity of eIF2B (state II).
(C) The presence of ISRIB hierarchically antagonizes the binding of eIF2(αP). Weaker antagonism toward one bound eIF2(αP) trimer (states I and III), as ISRIB’s binding pocket is less deformed under these circumstances. The stronger deformation of the ISRIB-binding pocket that is coupled to the binding of a second eIF2(αP) trimer sets up a competition whereby state IV is most strongly disfavored by ISRIB (rendered opaque in the cartoon). By stabilizing the ground state and the partially inhibited state III, ISRIB pulls the equilibrium away from the strongly inhibited state II, thus antagonizing the ISR. High enough concentrations of eIF2(αP) competitively override this inhibition.