Figure 4. Split SNARE-Driven Fusion Lacks Compartmental Specificity.
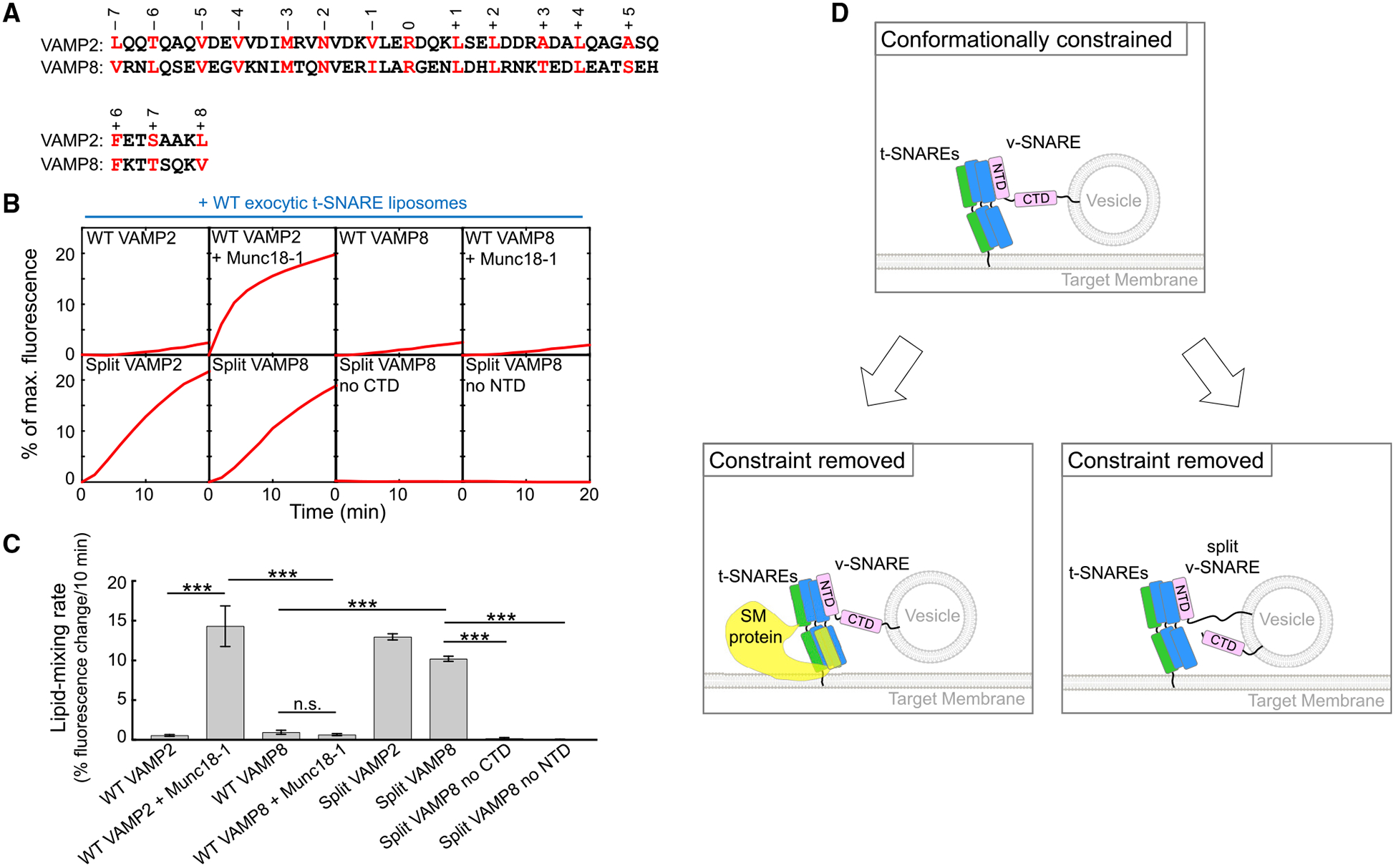
(A) Alignment of SNARE motifs of VAMP2 and VAMP8 with layer residues highlighted and numbered.
(B) Liposomes harboring the indicated v-SNAREs were directed to fuse with liposomes containing WT exocytic t-SNAREs (syntaxin-1 and SNAP-25) with or without 5 μM Munc18–1. The kinetics of the fusion reactions was measured by a FRET-based lipid-mixing assay.
(C) Initial lipid-mixing rates of the fusion reactions shown in (B). Data are presented as mean ± SD (n = 3). The p values were calculated using Student’s t test. n.s., p > 0.05. ***p < 0.001.
(D) Model illustrating the activation of the SNARE vesicle fusion machinery by a SM protein in a biological setting or by v-SNARE splitting in an engineered system. The CTDs of WT SNAREs are unable to zipper efficiently because of the presence of a conformational constraint. The SM protein uses its SLP to restructure t-SNARE CTDs, enabling the latter to properly zipper with the v-SNARE CTD. When the v-SNARE is split, the freed CTD is able to optimally zipper with t-SNARE CTDs, achieving the same effect as SM protein binding to t-SNAREs.
See also Figures S2–S4.
