Figure 4. MSBase and US chart review: (A and B) Post-treatment lymphocyte reconstitution for patients after discontinuing treatment with DMF through starting another DMT and (C) post-DMF DMTs.
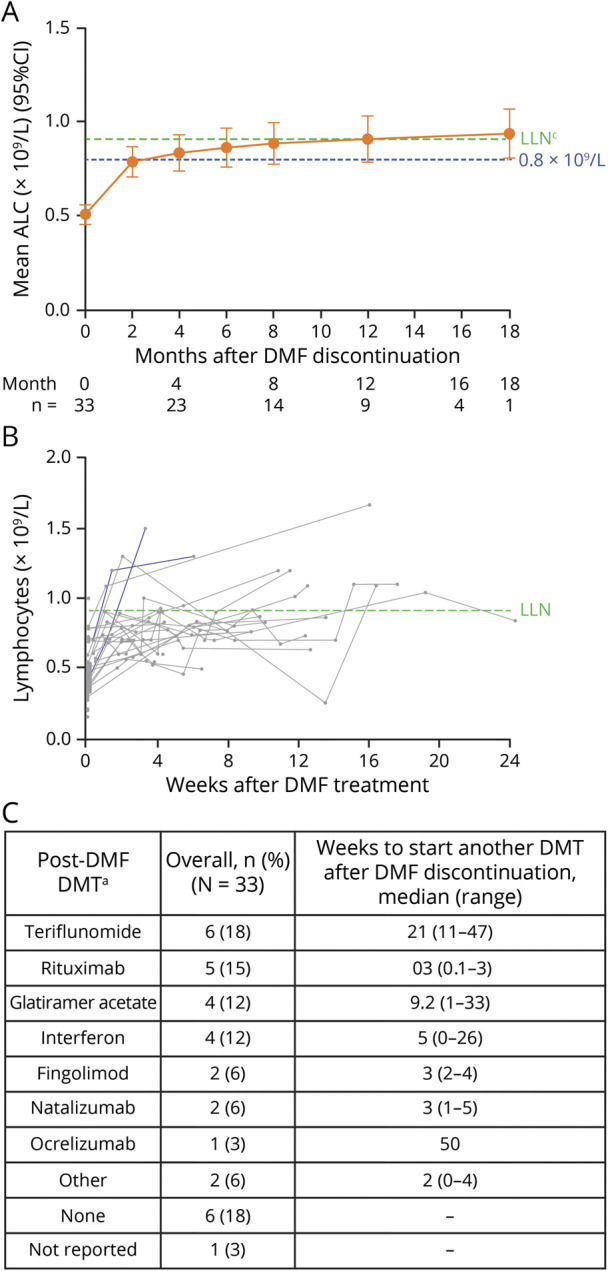
For patients who switched to fingolimod, natalizumab, rituximab, or ocrelizumab after DMF discontinuation, only the ALC observations taken before starting these treatments are included. aPatients could have sequentially switched to >1 DMT; only the patient's first alternative DMT after DMF discontinuation is shown. No patients reinstated DMF after discontinuation. (A) The mixed-effect model regresses absolute ALC measure on log2-transformed time (days) since discontinuation of DMF with random intercept and slope, adjusted for age at discontinuation, ALC group at discontinuation (<0.5 × 109/L or ≥0.5 × 109/L), and the interaction between the log2-transformed time and ALC group. The last ALC on DMF is assumed to be measured on day 1 after discontinuation. A month is assumed to be 30 days. The estimates at each time point are based on the fitted model with standard errors and CIs given using Kenward-Roger approximation. (B) ALC measures are shown for individual patients, and each patient's line ends when the patient begins an alternate therapy. ALC = absolute lymphocyte count; DMF = dimethyl fumarate; DMT = disease-modifying therapy; LLN = lower limit of normal.
