Figure 7.
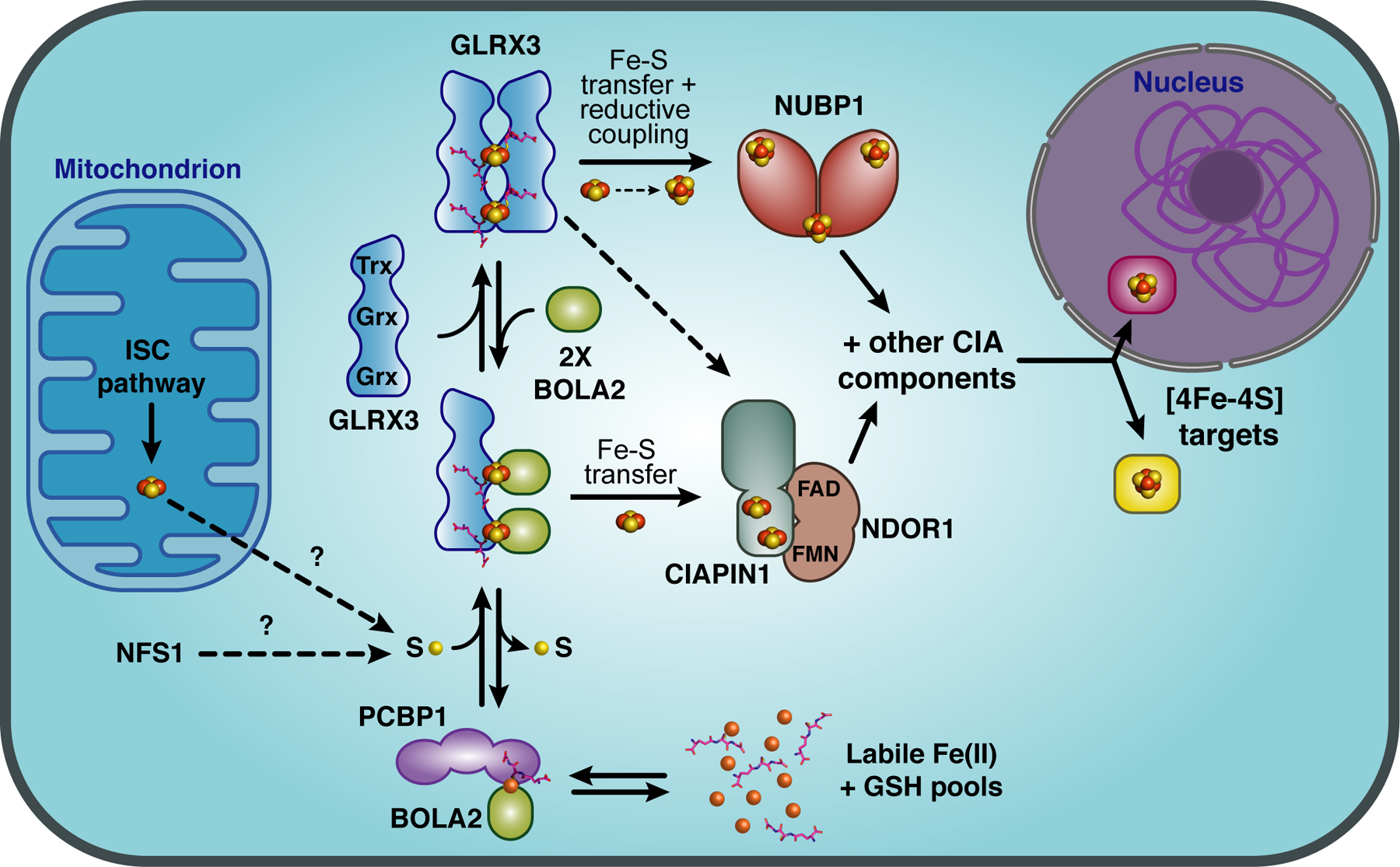
Model for the roles of GLRX3 and BOLA2 in mammalian cytosolic iron trafficking. The cytosolic iron chaperone PCBP1 acquires Fe(II) from the labile iron pool and forms an Fe(II)-bridged complex with BOLA2 that requires GSH binding for stability. This complex is proposed to interact with apo-GLRX3 to form a GLRX3-BOLA2 heterocomplex. Each Grx domain in GLRX3 forms a [2Fe-2S]-binding complex with a BOLA2 monomer and GSH leading to formation of a [2Fe-2S]2-bridged GLRX3-BOLA22-GSH2 heterotrimer. The sulfur source required to form the [2Fe-2S] clusters is unclear, possibly provided by the cytosolic isoform of the cysteine desulfurase NFS1 or exported from the mitochondrial ISC pathway. Both the [2Fe-2S]2-GLRX3-BOLA22 heterotrimer and the [2Fe-2S]2-GLRX32 homodimer can deliver [2Fe-2S] clusters to the CIA Fe-S cluster assembly protein CIAPIN1 in vitro, although both GLRX3 and BOLA2 are required for Fe incorporation into CIAPIN1 in cell-based assays. [2Fe-2S]2-GLRX32 may also deliver clusters to the CIA scaffold protein NUBP1, which requires reductive coupling of [2Fe-2S]2+ clusters to form [4Fe-4S]2+ clusters. CIAPIN1, its functional electron transfer partner NDOR1, and NUBP1 are all components of the CIA machinery that assembles and delivers [4Fe-4S] clusters to target proteins in the cytosol and nucleus.
