Abstract
Purpose
Perioperative anesthetic management may affect long-term outcome after cancer surgery. This study investigated the effect of perioperative glucocorticoids on long-term survival in patients after radical resection for pancreatic cancer.
Methods
In this retrospective cohort study with propensity score-matching, patients who underwent radical resection for pancreatic cancer from January 2005 to December 2016 were recruited. Baseline and perioperative data including use of glucocorticoids for prevention of postoperative nausea and vomiting were collected. Patients were followed up by qualified personnel for cancer recurrence and survival. The primary outcome was the recurrence-free survival. Outcomes were compared before and after propensity matching. The association between perioperative glucocorticoid use and recurrence-free survival was analyzed with multivariable regression models.
Results
A total of 215 patients were included in the study; of these, 112 received perioperative glucocorticoids and 103 did not. Patients were followed up for a median of 74.0 months (95% confidence interval [CI] 68.3–79.7). After propensity score-matching, 64 patients remained in each group. The recurrence-free survivals were significantly longer in patients with glucocorticoids than in those without (full cohort: median 12.0 months [95% CI 6.0–28.0] vs 6.9 months [4.2–17.0], P<0.001; matched cohort: median 12.0 months [95% CI 5.8–26.3] vs 8.3 months [4.3–18.2], P=0.015). After correction for confounding factors, perioperative glucocorticoids were significantly associated with prolonged recurrence-free survivals (full cohort: HR 0.66, 95% CI 0.48–0.92, P=0.015; matched cohort: HR 0.54, 95% CI 0.35–0.84, P=0.007).
Conclusion
Perioperative use of low-dose glucocorticoids is associated with improved recurrence-free survival in patients following radical surgery for pancreatic cancer.
Keywords: pancreatic cancer, surgery, perioperative management, glucocorticoids, survival
Introduction
Pancreatic cancer is a malignant tumor with high mortality. According to the latest Global Cancer Statistics, 459,000 new cases of pancreatic cancer were diagnosed and 432,000 cases died in 2018, ranking as the seventh leading cause of cancer death worldwide.1 According to the Chinese data, 90,100 new cases of pancreatic cancer were diagnosed and 79,400 cases died in 2015.2 Currently, surgical resection accompanied by systemic adjuvant chemotherapy is the only possible treatment for patients to achieve prolonged survival.3 However, recent evidence showed that the clinical outcome of pancreatic cancer patients remains poor even after radical resection and modern adjuvant chemotherapy, with a median overall survival of 28–54 months.4 Recurrence and metastasis are the main reasons leading to short survival after pancreatic cancer surgery. This is because that the pancreatic cancer cells invade the lymphatic system and form micro-metastases at the very early stage, which make radical resection incomplete.4 Furthermore, surgery-related inflammation may also promote tumor growth and metastasis.5
Anesthetic management may affect the prognosis of cancer patients by regulating perioperative immunity and inflammation. For example, epidural block, dexmedetomidine, and non-steroidal anti-inflammatory drugs can blunt surgery-related stress response6,7 and, thus, may provide benefits for long-term outcome of some cancer patients.8–10 Low-dose glucocorticoids (dexamethasone or methylprednisolone) are frequently used to prevent postoperative nausea and vomiting;11 in addition, they have transient effects on immune function and inflammatory response.12 In animal studies of pancreatic cancer, inflammation increases the dissemination of cancer cells whereas dexamethasone abolishes this phenomenon.13 In two retrospective studies, perioperative low-dose dexamethasone was found to be associated with improved survival after pancreatic cancer surgery.14,15 Our study of patients undergoing lung cancer surgery also revealed that perioperative use of low-dose dexamethasone was associated with improved long-term survival.16 However, the effects of perioperative glucocorticoids on cancer outcome are conflicting; some authors even reported negative results, ie, worsened long-term survival.17–19
Considering the popularity of glucocorticoid use in the perioperative period, it is necessary to further evaluate its effect on the long-term outcome of cancer patients. The purpose of this propensity-matched retrospective study was to analyze the association between perioperative glucocorticoid use and long-term survival in patients with pancreatic cancer.
Materials and Methods
This was a retrospective cohort study with propensity score-matching. The study protocol was approved by the Clinical Research Ethics Committee of Peking University Cancer Hospital (2018YJZ49). This study was conducted in accordance with the Declaration of Helsinki. Considering that the study was pure observational, the Ethics Committee agreed to waive written informed consents; however, all patients or their family members had provided oral consents to participate in this study before collecting data.
Participants
Potential participants were screened using the electronic medical record system of the hospital. Inclusion criteria were patients who underwent radical resection for pancreatic cancer of which the diagnoses were confirmed by pathological examination from January 1, 2005 to December 31, 2016 in the first Department of Hepatic, Biliary & Pancreatic Surgery of Peking University Cancer Hospital. Exclusion criteria included the following: (1) combined primary cancer in other sites; (2) recurrent or metastatic pancreatic cancer; (3) long-term glucocorticoid therapy before surgery; (4) non-radical surgery; or (5) missing data (such as cancer size, stage, differentiation, and follow-up data, etc.).
Anesthesia, Surgery and Perioperative Management
All patients underwent general anesthesia with endotracheal intubation. Anesthesia was induced with intravenous anesthetics (propofol and/or etomidate) and opioids (fentanyl or sufentanil), and maintained with inhalational anesthetics (sevoflurane or isoflurane) and opioids (fentanyl, sufentanil, oxycodone, and/or dezocine). For some patients, non-steroidal anti-inflammatory drugs (NSAIDs, including flurbiprofen axetil and parecoxib) were administered for supplemental analgesia; epidural anesthesia was performed with local anesthetics (lidocaine and/or ropivacaine) for anesthesia maintenance and postoperative analgesia. Low-dose glucocorticoids, either dexamethasone or methylprednisolone, were administered to prevent postoperative nausea and vomiting depending on the discretion of anesthesiologists.11 There were no special indications for glucocorticoid administration during the study period.
The standard median incision approach was adopted for radical resection of pancreatic cancer. Surgical procedures were performed by three chief surgeons until the end of April 2007, and by one chief surgeon thereafter. The types of surgery were decided according to the status of cancer at the discretion of the surgeons, and included pancreaticoduodenectomy, pancreatic body and tail resection plus splenectomy, and total pancreatectomy. The range of lymph node dissection was standardized for each type of surgery. Positive surgical margin was defined when residual cancer cells were found within 1 mm of the surgical resection margins.
Postoperative patient-controlled analgesia was provided for up to 3 days. Opioids (with or without flurbiprofen axetil) were used for intravenous analgesia. Ropivacaine (with or without opioids) was used for epidural analgesia. Antiemetics including dexamethasone, 5-HT3 receptor antagonist, and/or metoclopramide were administered when considered necessary.20 Other perioperative treatments were performed according to routine practice.
Perioperative Data Collection
Patients’ data were collected from the hospital’s electronic medical record system. Baseline data included age, sex, height, weight, preoperative comorbidities, preoperative laboratory test results, ASA classification, and preoperative chemotherapy. Anesthesia-related data included anesthetic method, duration of anesthesia, types and doses of anesthetics, intraoperative fluid infusion and blood transfusion, postoperative analgesia, as well as perioperative use of glucocorticoids and non-steroidal anti-inflammatory drugs. Equivalent doses were calculated for opioids, NSAIDs, and glucocorticoids.21–25 Surgery-related data included date and type of surgery, surgical margin status, and estimated intraoperative blood loss. Postoperative data included pathological diagnoses, maximum tumor diameter, degree of cancer differentiation, Tumor-Node-Metastasis (TNM) stage of pancreatic cancer (pTNM stage), occurrence of postoperative complications, length of hospital stay, and in-hospital death.
Postoperative Long-Term Follow-Up
Patients were followed up by surgeons and specially assigned personnel after surgery. Follow-ups were performed every 6 months during the first year and once a year thereafter, in the way of outpatient review, telephone inquiry, or letter communication. During each follow-up, the results of re-examinations, the acceptance of radio-/chemotherapies and the status of living were recorded. Cancer recurrence referred to local recurrence and/or distant metastasis as confirmed by imagological examinations.26 For those with cancer recurrence, the time of diagnosis was recorded; and for those who died, the time and cause of death were documented. The time of recurrence was the earliest date of imagological evidence according to which clinical diagnosis was made by surgeons. The time of death was extracted from the medical death certificate. The recurrence-free survival and overall survival were determined according to follow-up results. Postoperative follow-up continued until patients died, lost to follow-up, or end of follow-ups.
Outcomes
The primary outcome was recurrence-free survival, ie, the duration from date of surgery until confirmed recurrence, all-cause death, loss to follow-up or end of follow-up, whichever came first. Secondary outcomes included occurrence of postoperative complications, length of stay in hospital after surgery, in-hospital mortality, and overall survival. Postoperative complications were defined as newly occurred medical events that were harmful to the patients’ recovery during hospital stay and required interventional therapy, ie, class II–V on Clavien-Dindo classification.27 Overall survival was defined as the duration from date of surgery until all-cause death, loss to follow-up, or end of follow-up, which ever came first.
Statistical Analysis
Sample Size Estimation
In a previous study, the median overall survival in patients with and without perioperative glucocorticoids was 46 and 22 months, respectively.15 We expected a total recruitment period of 144 months and a total follow-up period of 168 months (ie, 2 more years after the last recruitment), respectively. With the significance level set at 0.05, power at 0.8, and drop-out rate at 5%, a minimum of 99 and 98 subjects in the glucocorticoid and no-glucocorticoid groups, respectively, was needed to detect the difference. Sample size estimation was performed with the Survival-Log rank-Lakatos-Median Survival Time of the PASS 11.0 (NCSS LLC, Kaysville, UT, USA).
Data Analysis
For the purpose of analyses, patients were divided into two groups, ie, those with glucocorticoids during the intra-/postoperative period (from the day of surgery to the 3rd day after surgery) and those without. Between-group differences of baseline and intra-/postoperative variables used for propensity score-matching were compared using the absolute standardized differences (ASDs), which are defined as the absolute difference in means, mean ranks, or proportions divided by the pooled standard deviation and calculated with the formula published by Austin.28 An ASD ≥0.219, ie, 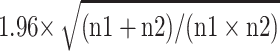 , was considered imbalanced between the two groups. Regarding variables not used for propensity score matching, continuous data were compared using the Student’s t-test (normal distribution) or Mann–Whitney U-test (non-normal distribution); categorical data were analyzed using the chi-square test. Missing data were not replaced.
, was considered imbalanced between the two groups. Regarding variables not used for propensity score matching, continuous data were compared using the Student’s t-test (normal distribution) or Mann–Whitney U-test (non-normal distribution); categorical data were analyzed using the chi-square test. Missing data were not replaced.
Variables that were considered clinically relevant were used for propensity score matching and were selected a priori. Baseline data included age, sex, body mass index, ASA classification, Charlson Comorbidity Index,29 preoperative laboratory test results, preoperative chemotherapy, pathological diagnoses, maximum tumor diameter, Tumor-Node-Metastasis (TNM) stage of pancreatic cancer (pTNM stage), and degree of cancer differentiation. Intra- and postoperative data included date of surgery, type of surgery, status of surgical margin, duration of surgery, type of anesthesia, estimated blood loss, blood transfusion, perioperative NSAIDs, postoperative radiotherapy and chemotherapy, as well as duration of long-term follow-up. Patients were matched in a 1:1 ratio using the nearest-neighbor matching with caliper widths equal to 0.2 of the standard deviation of the logit of the propensity score.
For both full cohort and matched cohort, time-to-event variables (recurrence-free survival, overall survival, and hospital stay after surgery) were analyzed with Kaplan-Meier estimator, with differences between groups assessed with Log-rank tests. Patients who were lost during follow-up were censored at the time of last follow-ups. Categorical variables (postoperative complications, in-hospital death rate, recurrence/death rate during follow-up, and all-cause death rate during follow-up) were compared with the χ2 test. Missing data were not replaced. Univariable associations between baseline/perioperative variables and recurrence-free/overall survival were performed with cox proportional hazard regression models. Those with P<0.20 in univariate analyses and those that were considered clinically important were included in multivariable models to assess the adjusted association between perioperative glucocorticoid use and recurrence-free/overall survival.
Statistical analyses were performed with the SPSS 25.0 software (IBM SPSS Inc, Chicago, IL) and the free software package “R”version 2.15.3 including the “Matchit” and the “ROC” plugin. A two-sided P<0.05 was considered statistically significant.
Results
Patient Recruitment
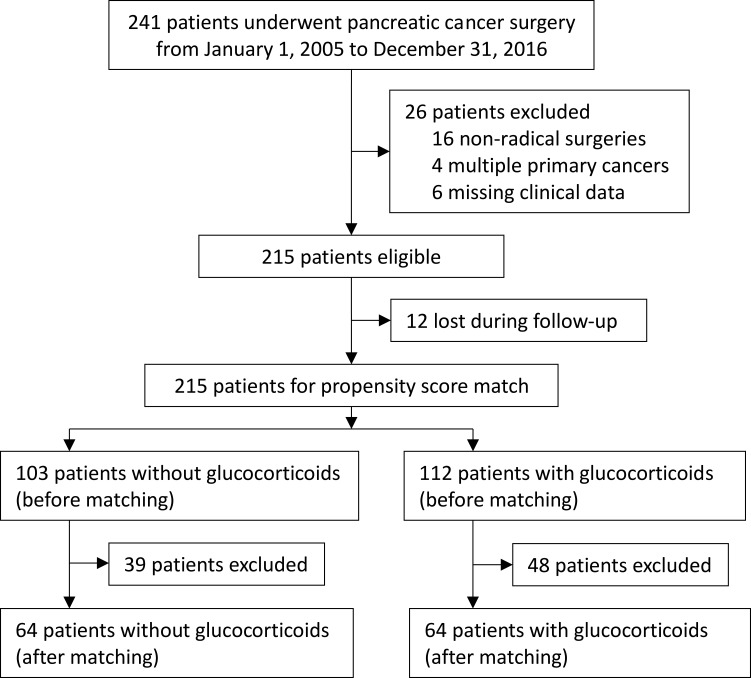
A total of 241 patients underwent surgery for pancreatic cancer between January 1, 2005 and December 31, 2016. Of these, 26 cases were excluded after data review, including 16 non-radical surgeries, 4 multiple primary cancers, and 6 with missing data (1 no pathological cancer stage, 1 no tumor differentiation grade, and 4 no follow-up data). Postoperative follow-ups were ended on August 12, 2018. Of the recruited 215 patients, 12 were lost during follow-up. All 215 cases were included in final analyses; of these, 112 received perioperative glucocorticoids and 103 did not. After propensity-matching, 64 patients remained in each group (Figure 1; Supplement Table 1).
Figure 1.
Flowchart of the study.
Baseline and Perioperative Data
In the full cohort, when compared with patients without glucocorticoids, those who received glucocorticoids were younger, underwent surgeries in the later period, had more negative margin, received less intraoperative crystalloid infusion, received more postoperative chemotherapy, and were followed up for a shorter duration. After propensity-matching, the two groups were well-balanced regarding baseline and perioperative variables (Tables 1 and 2; Supplement Table 2 and Supplement Table 3).
Table 1.
Baseline and Perioperative Data Used for Propensity Score Matching
| Variables | All Patients (n=215) | Full Cohort (n=215) | Matched Cohort (n=128) | ||||
|---|---|---|---|---|---|---|---|
| With Glucocorticoids (n=112) | Without Glucocorticoids (n=103) | ASDa | With Glucocorticoids (n=64) | Without Glucocorticoids (n=64) | ASDa | ||
| Age (year) | 63 (55–70) | 62 (53–69) | 65 (58–71) | 0.288 | 64 (54–73) | 63 (56–68) | 0.060 |
| Male sex | 125 (58.1%) | 59 (52.7%) | 66 (64.1%) | 0.227 | 35 (54.7%) | 39 (60.9%) | 0.136 |
| Body mass index (kg/m2) | 22.9 (21.3–24.8) | 23.3 (21.4–24.7) | 22.8 (21.2–25.1) | 0.059 | 23.4 (21.8–25.2) | 23.3 (21.0–25.2) | 0.052 |
| ASA grade | 0.099 | 0.063 | |||||
| I | 26 (9.8%) | 11 (5.4%) | 15 (14.6%) | 6 (9.4%) | 5 (7.8%) | ||
| II | 166 (81.9%) | 88 (87.5%) | 78 (75.7%) | 54 (83.1%) | 54 (83.1%) | ||
| III | 23 (8.4%) | 13 (7.1%) | 10 (9.7%) | 4 (6.3%) | 5 (7.8%) | ||
| Charlson Comorbidity Indexb | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.081 | 3 (2–3) | 3 (2–3) | 0.048 |
| Preoperative lab test | |||||||
| CA19-9 (U/mL) | 238.9 (59.9–658.7) | 185.6 (43.2–589.9) | 275.1 (95.4–845.6) | 0.012 | 170.6 (43.4–569) | 276.2 (74.9–689.8) | 0.102 |
| Albumin (g/L) | 39 (38–41) | 39 (37–41) | 39 (38–41) | 0.090 | 39 (37–41) | 39 (38–40) | 0.079 |
| Hemoglobin (g/L) | 127 (122–131) | 128 (123–132) | 127 (121–131) | 0.158 | 128 (123–131) | 127.5 (121.0–131.0) | 0.056 |
| Preoperative chemotherapy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – | 0 (0.0%) | 0 (0.0%) | – |
| Pathological diagnosis | 0.134 | 0.000 | |||||
| Ductal adenocarcinoma | 203 (94.4%) | 107 (95.5%) | 96 (93.2%) | 62 (96.9%) | 62 (96.9%) | ||
| Intraductal papilloma | 2 (0.9%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Adenosquamous carcinoma | 1 (0.5%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Neuroendocrine tumor | 3 (1.4%) | 2 (1.8%) | 1 (1.0%) | 1 (1.6%) | 1 (1.6%) | ||
| Mucinous adenocarcinoma | 6 (2.8%) | 1 (0.9%) | 5 (4.9%) | 1 (1.6%) | 1 (1.6%) | ||
| Maximum diameter (mm) | 30 (25–40) | 30 (24–39) | 32 (25–42) | 0.042 | 31 (24–39) | 33 (25–40) | 0.139 |
| Pathologic TNM stagec | 0.192 | 0.044 | |||||
| IA | 4 (1.9%) | 3 (2.7%) | 1 (1.0%) | 1 (1.6%) | 1 (1.6%) | ||
| IB | 16 (7.4%) | 10 (8.9%) | 6 (5.8%) | 6 (9.4%) | 5 (7.8%) | ||
| IIA | 71 (33.0%) | 42 (37.5%) | 29 (28.2%) | 22 (34.4%) | 23 (35.9%) | ||
| IIB | 103 (47.9%) | 48 (42.9%) | 55 (53.4%) | 32 (50.0%) | 31 (48.4%) | ||
| III | 17 (7.9%) | 9 (8.0%) | 8 (7.8%) | 3 (4.7%) | 4 (6.3%) | ||
| IV | 4 (1.9%) | 0 (0.0%) | 4 (3.9%) | 0 (0.0%) | 0 (0.0%) | ||
| Differentiation grade | 0.134 | 0.103 | |||||
| Low | 92 (42.8%) | 45 (40.2%) | 47 (45.6%) | 30 (46.9%) | 29 (45.3%) | ||
| Moderate | 116 (54.0%) | 64 (57.1%) | 52 (50.5%) | 31 (48.4%) | 33 (51.6%) | ||
| High | 7 (3.3%) | 3 (2.7%) | 4 (3.9%) | 3 (4.7%) | 2 (3.1%) | ||
| Period of surgery | 0.536 | 0.063 | |||||
| 2005-April 2007 | 35 (16.3%) | 10 (8.9%) | 25 (24.3%) | 6 (9.4%) | 5 (7.8%) | ||
| May 2007–2012 | 100 (46.5%) | 47 (42.0%) | 53 (51.5%) | 35 (54.7%) | 38 (59.4%) | ||
| 2013–2016 | 80 (37.2%) | 55 (49.1%) | 25 (24.3%) | 23 (35.9%) | 21 (32.8%) | ||
| Type of surgery | 0.118 | 0.080 | |||||
| Pancreaticoduodenectomy | 150 (69.8%) | 80 (71.4%) | 70 (68.0%) | 45 (70.3%) | 43 (67.2%) | ||
| Distal pancreatosplenectomy | 61 (28.4%) | 29 (25.9%) | 32 (31.1%) | 18 (28.1%) | 20 (31.3%) | ||
| Total pancreatectomy | 4 (1.9%) | 3 (2.7%) | 1 (1.0%) | 1 (1.6%) | 1 (1.6%) | ||
| Pathological negative margin | 190 (88.4%) | 103 (92.0%) | 87 (84.5%) | 0.275 | 59 (92.2%) | 59 (92.2%) | 0.068 |
| Duration of surgery (min) | 300 (226–366) | 300 (221–357) | 300 (234–369) | 0.123 | 300 (202–338) | 298 (224–369) | 0.102 |
| Type of anesthesia | 0.062 | 0.130 | |||||
| General | 200 (93.0%) | 105 (93.8%) | 95 (92.2%) | 60 (93.8%) | 62 (96.9%) | ||
| Combined epidural-general | 15 (7.0%) | 7 (6.3%) | 8 (7.8%) | 4 (6.3%) | 2 (3.1%) | ||
| Estimated blood loss (mL) | 300 (200–500) | 200 (160–400) | 300 (200–600) | 0.106 | 200 (160–400) | 300 (200–575) | 0.038 |
| Blood transfusion | 48 (22.3%) | 21 (18.8%) | 27 (26.2%) | 0.190 | 11 (17.2%) | 13 (20.3%) | 0.050 |
| Perioperative NSAIDsd | 130 (60.5%) | 67 (59.8%) | 63 (61.2%) | 0.027 | 43 (67.2%) | 45 (70.3%) | 0.034 |
| Postoperative therapy | |||||||
| Radiotherapy | 22 (10.2%) | 14 (12.5%) | 8 (7.8%) | 0.142 | 5 (7.8%) | 7 (10.9%) | 0.099 |
| Chemotherapy | 103 (47.9%) | 61 (54.5%) | 42 (40.8%) | 0.274 | 29 (45.3%) | 31 (48.4%) | 0.078 |
| Duration of follow-up (month) | 74.0 (68.3–79.7) | 65.7 (58.4–73.0) | 93.8 (85.5–102.1) | 0.498 | 72.6(40.9–108.1) | 79.5 (46.3–105.2) | 0.098 |
Notes: aAn ASD ≥0.219 was considered unbalanced28. bCalculated according to the version without age.29 cAccording to the 8th Edition of the American Joint Committee on Cancer/Union for International Cancer Control Staging System. dIncludes flurbiprofen axetil and parecoxib sodium. Data are presented as median (interquartile range) or number (%). ASDs in bold indicate those of ≥0.219.
Abbreviations: ASA, American Society of Anesthesiologists; CA, cancer antigen; TNM stage, tumor-node-metastasis stage; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 2.
Baseline and Perioperative Data Not Used for Propensity Score Matching
| Variables | All Patients (n=215) | Full Cohort (n=215) | Matched Cohort (n=128) | ||||
|---|---|---|---|---|---|---|---|
| With Glucocorticoids (n=112) | Without Glucocorticoids (n=103) | P value | With Glucocorticoids (n=64) | Without Glucocorticoids (n=64) | P value | ||
| Preoperative comorbidities | |||||||
| Previous stroke | 8 (3.7%) | 6 (5.4%) | 2 (1.9%) | 0.283 | 4 (6.3%) | 1 (1.6%) | 0.365 |
| Coronary heart disease | 15 (7.0%) | 4 (3.6%) | 11 (10.7%) | 0.059 | 3 (4.7%) | 8 (12.5%) | 0.206 |
| Hypertension | 65 (30.2%) | 29 (25.9%) | 36 (35.0%) | 0.181 | 16 (25.0%) | 23 (35.9%) | 0.249 |
| Arrhythmia | 47 (21.9%) | 24 (21.4%) | 23 (22.3%) | >0.999 | 17 (26.6%) | 17 (26.6%) | >0.999 |
| COPDa | 15 (7.0%) | 10 (8.9%) | 5 (4.9%) | 0.291 | 7 (10.9%) | 3 (4.7%) | 0.324 |
| Asthma | 5 (2.3%) | 1 (0.9%) | 4 (3.9%) | 0.196 | 0 (0.0%) | 2 (3.1%) | 0.496 |
| Old tuberculosis | 4 (1.9%) | 2 (1.8%) | 2 (1.9%) | >0.999 | 0 (0.0%) | 1 (1.6%) | >0.999 |
| Diabetes | 48 (22.3%) | 27(24.1%) | 21 (20.4%) | 0.623 | 13 (20.3%) | 15 (23.4%) | 0.831 |
| Renal dysfunctionb | 1 (0.5%) | 0 (0.0%) | 1 (1.0%) | 0.479 | 0 (0.0%) | 0 (0.0%) | >0.999 |
| Liver dysfunctionc | 102 (47.4%) | 57 (50.9%) | 45 (43.7%) | 0.339 | 32 (50.0%) | 29 (45.3%) | 0.724 |
| Duration of anesthesia (min) | 332 (267–402) | 334 (265–386) | 331 (270–409) | 0.491 | 332 (240.5–384) | 325.5 (256–403) | 0.567 |
| Intravenous anesthetics | |||||||
| Propofol dose (mg) | 150 (100–160) | 150 (100–160) | 150 (50–180) | 0.436 | 150 (100–160) | 140 (50–180) | 0.102 |
| Etomidate dose (mg) | 0 (0–10) | 0 (0–10) | 0 (0–15) | 0.147 | 0 (0–10) | 5 (0–18) | 0.056 |
| Intraoperative fluid | |||||||
| Crystalloids (mL) | 1800 (1400–2250) | 1625 (1300–2200) | 2000 (1500–2600) | 0.004 | 1700 (1260–2075) | 1825 (1500–2450) | 0.063 |
| Artificial colloid (mL)d | 1000 (1000–1500) | 1000 (1000–1500) | 1000 (800–1500) | 0.806 | 1000 (1000–1500) | 1000 (650–1000) | 0.184 |
| Blood transfused (mL) | 0 (0–0) | 0 (0–0) | 0 (0–400) | 0.197 | 0 (0–0) | 0 (0–0) | 0.712 |
| Perioperative medication | |||||||
| Inhalational anesthetics | 215 (100.0%) | 112 (100%) | 103 (100%) | — | 64 (100%) | 64 (100%) | — |
| Sufentanil equivalent, µge | 255 (120–320) | 270 (140–320) | 255 (85–320) | 0.093 | 252.5 (138.5–320) | 252.5 (153–323) | 0.791 |
| FA equivalent, mgf | 100 (0–300) | 100 (0–387.5) | 100 (0–300) | 0.983 | 250 (0–400) | 300 (0–300) | 0.918 |
| Glucocorticoidsg | 112 (52.1%) | 112 (100.0%) | — | — | 64 (100.0%) | — | — |
| DXM equivalent (mg)h | 5 (0–10) | 10 (6.75–10) | — | — | 10 (10–10) | — | — |
Notes: Data are median (interquartile range) or number (%). P values in bold indicate those of <0.05. aInclude chronic bronchitis and emphysema. bSerum creatinine >133µmol/L. cAlanine transaminase, aspartate transaminase and/or total bilirubin 2 times higher than the upper normal limit. dIncludes hydroxyethyl starch 130/0.4, hydroxyethyl starch 200/0.5, and succinylated gelatin. e1 mg morphine (iv) = 15 µg fentanyl = 1.5 µg sufentanil = 1 mg oxycodone = 1 mg dezocine.21–23 fIncludes flurbiprofen axetil and parecoxib sodium. 40 mg parecoxib sodium = 50 mg flurbiprofen axetil.24 gIncludes methylprednisolone and dexamethasone. h1 mg methylprednisolone = 0.2 mg dexamethasone.25
Abbreviations: FA, flurbiprofen axetil; DXM, dexamethasone.
Postoperative Outcomes
Of all patients, the estimated 5-year recurrence-free and overall survival rates were 10.8% (95% CI 6.5–15.1) and 11.1% (95% CI 6.2–16.0), respectively. In both the full cohort and the matched cohort, recurrence-free survivals were significantly longer in patients with glucocorticoids than in those without (full cohort: median 12.0 months [95% CI 6.0–28.0] vs 6.9 months [4.2–17.0], P<0.001; matched cohort: 12.0 months [5.8–26.3] vs 8.3 months [4.3–18.2], P=0.015) (Figure 2A and C). As expected, the recurrence/death rate during follow-up was significantly lower in patients with glucocorticoids than in those without (full cohort: 91 (81.3%) vs 98 (95.1%), P=0.003; matched cohort: 52 (81.3%) vs 61 (95.3%), P=0.025). Overall survival was significantly longer in patients with glucocorticoids than in those without only in the full cohort (median 19.7 months [12.3–36.2] vs 13.9 months [8.0–23.9], P<0.001) (Figure 2B), but not in the matched cohort (Figure 2D). Perioperative outcomes including postoperative complications, length of hospital stay, and in-hospital mortality did not differ between the two groups in both cohorts (Table 3; Supplement Table 4 and Supplement Table 5).
Figure 2.
Survival curves of patients with or without perioperative glucocorticoids after pancreatic cancer surgery. In the full cohort, the recurrence-free survival (A) and overall survival (B) were significantly longer in patients with glucocorticoids than in those without (Log-rank test, both P<0.001). In the matched cohort, the recurrence-free survival (C) was significantly longer in patients with glucocorticoids than in those without (Log-rank test P=0.015); the overall survival (D) did not differ significantly between the two groups. +, subjects who were censored.
Table 3.
Postoperative and Long-Term Outcomes
| Variables | All Patients (n=215) | Full Cohort (n=215) | Matched cohort (n=128) | ||||
|---|---|---|---|---|---|---|---|
| With Glucocorticoids (n=112) | Without GlucocortIcoids (n=103) | P value | With GlucocortIcoids (n=64) | Without GlucocortIcoids (n=64) | P value | ||
| Primary endpoint | |||||||
| Recurrence-free survival (month)a | 9.4 (5.1–20.3) | 12.0 (6.0–28.0) | 6.9 (4.2–17.0) | <0.001 | 12.0 (5.8–26.3) | 8.3 (4.3–18.2) | 0.015 |
| Secondary endpoints | |||||||
| Postoperative complicationsb | 51 (23.7%) | 22 (19.6%) | 29 (28.2%) | 0.152 | 14 (21.9%) | 19 (29.7%) | 0.419 |
| Length of hospital stay (day) | 16.0 (8.0–27.0) | 16.0 (13.4–18.6) | 17.0 (11.3–22.7) | 0.754 | 17.0 (12.7–21.3) | 13.0 (8.8–17.2) | 0.360 |
| In-hospital death | 2 (0.9%) | 1 (1.0%) | 1 (0.9%) | >0.999 | 0 (0.0%) | 0 (0.0%) | — |
| Recurrence/death during follow-upa | 189 (87.9%) | 91 (81.3%) | 98 (95.1%) | 0.003 | 52 (81.3%) | 61 (95.3%) | 0.025 |
| All-cause death during follow-upc | 182 (84.7%) | 90 (80.4%) | 92 (89.3%) | 0.088 | 51 (79.7%) | 56 (87.5%) | 0.340 |
| Overall survival (month)c | 16.8 (10.4–29.2) | 19.7 (12.3–36.2) | 13.9 (8.0–23.9) | <0.001 | 17.2 (11.3–31.9) | 14.7 (9.0–27.7) | 0.106 |
Notes: Data are median (95% CI), number (%), or median (interquartile range). P values in bold indicate those of <0.05. aLocal recurrence and/or distant metastasis as confirmed by imaging examination,26 or all-cause death.b Newly occurred medical events that were harmful to the patients’ recovery during postoperative hospitalization and required interventional therapy, ie, class II–V on Clavien-Dindo classification. cAll-cause death.
Perioperative Glucocorticoids and Recurrence-Free Survival
In the full cohort, 16 factors were included in the multivariable model, including 12 factors with P<0.20 in univariable analyses (perioperative use of glucocorticoids, age ≥70 y, preoperative hepatorenal dysfunction, preoperative CA19-9 level, ductal adenocarcinoma, higher pTNM stage, low differentiation grade, positive surgical margin, estimated blood loss ≥400 mL, period of surgery, postoperative complications, and postoperative chemotherapy) and 4 factors that were considered clinically important (BMI, type of surgery, type of anesthesia, and type of inhalational anesthetics). After correction for confounding factors, perioperative glucocorticoid use was significantly associated with a prolonged recurrence-free survival (HR 0.66, 95% CI 0.48–0.92, P=0.015). Among other factors, ductal adenocarcinoma (vs others), higher pTNM stage (IIB, III, IV vs IA, IB, IIA), low differentiation grade (vs medium/high grade), positive surgical margin, and estimated blood loss ≥400 mL were associated with shortened recurrence-free survival (Table 4; Supplement Table 6).
Table 4.
Cox Regression Proportional Hazard Survival: Multivariable Model for Full Cohort and Matched Cohort
| Factorsa | Full Cohort (n=215) | Matched Cohort (n=128) | ||||||
|---|---|---|---|---|---|---|---|---|
| Recurrence-Free Survival | Overall Survival | Recurrence-Free Survival | Overall Survival | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Perioperative glucocorticoidsb | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.66 (0.48–0.92) | 0.015 | 0.75 (0.53–1.04) | 0.084 | 0.54 (0.35–0.84) | 0.007 | 0.70 (0.45–1.11) | 0.127 |
| Age (y) | ||||||||
| <60 | 1 | 1 | 1 | 1 | ||||
| 60–69 | 0.93 (0.65–1.34) | 0.701 | 1.01 (0.70–1.46) | 0.950 | 0.85 (0.52–1.40) | 0.528 | 0.88 (0.53–1.47) | 0.628 |
| ≥70 | 1.28 (0.86–1.90) | 0.232 | 1.33 (0.89–2.00) | 0.161 | 1.94 (1.11–3.38) | 0.020 | 1.72 (0.97–3.05) | 0.062 |
| Body mass index (kg/m2) | ||||||||
| ≤24.9 | 1 | 1 | 1 | 1 | ||||
| ≥25 | 0.79 (0.55–1.13) | 0.190 | 0.83 (0.57–1.21) | 0.336 | 0.67 (0.41–1.09) | 0.107 | 0.71 (0.42–1.18) | 0.185 |
| Hepatorenal dysfunction | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.08 (0.74–1.58) | 0.702 | 1.10 (0.75–1.61) | 0.642 | 1.87 (1.10–3.19) | 0.021 | 1.36 (0.81–2.28) | 0.248 |
| Preoperative CA19-9 | ||||||||
| <240 U/mL | 1 | 1 | 1 | 1 | ||||
| ≥240 U/mL | 1.28 (0.93–1.76) | 0.126 | 1.07 (0.78–1.48) | 0.672 | 1.34 (0.86–2.08) | 0.202 | 1.37 (0.86–2.18) | 0.183 |
| Pathological diagnosis | ||||||||
| Othersc | 1 | 1 | 1 | 1 | ||||
| Ductal adenocarcinoma | 2.55 (1.14–5.68) | 0.022 | 2.09 (0.98–4.46) | 0.058 | 5.68 (1.43–22.73) | 0.014 | 6.06 (1.61–22.73) | 0.008 |
| pTNM staged | ||||||||
| IA, IB, IIA | 1 | 1 | 1 | 1 | ||||
| IIB, III, IV | 1.50 (1.08–2.09) | 0.016 | 1.68 (1.19–2.39) | 0.004 | 1.79 (1.16–2.77) | 0.009 | 1.59 (1.01–2.50) | 0.045 |
| Differentiation grade | ||||||||
| Moderate and high | 1 | 1 | 1 | 1 | ||||
| Low | 2.15 (1.53–3.02) | <0.001 | 2.01 (1.43–2.84) | <0.001 | 1.98 (1.25–3.13) | 0.003 | 1.75 (1.09–2.80) | 0.021 |
| Period of surgery | ||||||||
| 2005-April 2007 | 1 | 1 | 1 | 1 | ||||
| May 2007–2012 | 1.09 (0.63–1.89) | 0.763 | 0.98 (0.57–1.68) | 0.937 | 0.75 (0.30–1.84) | 0.524 | 0.93 (0.39–2.25) | 0.879 |
| 2013–2016 | 1.14 (0.61–2.12) | 0.682 | 0.78 (0.43–1.41) | 0.413 | 0.55 (0.21–1.48) | 0.238 | 0.54 (0.20–1.45) | 0.223 |
| Type of surgery | ||||||||
| Pancreaticoduodenectomy/ total pancreatectomy |
1 | 1 | 1 | 1 | ||||
| Distal pancreatosplenectomy | 0.82 (0.50–1.34) | 0.435 | 0.77 (0.48–1.23) | 0.271 | 1.27 (0.69–2.33) | 0.444 | 0.96 (0.53–1.76) | 0.902 |
| Margin status | ||||||||
| Negative | 1 | 1 | 1 | 1 | ||||
| Positive | 1.70 (1.00–2.90) | 0.049 | 1.95 (1.14–3.36) | 0.015 | 2.74 (1.31–5.70) | 0.007 | 2.74 (1.24–6.02) | 0.012 |
| Type of anesthesia | ||||||||
| General | 1 | 1 | 1 | 1 | ||||
| Combined | 0.69 (0.33–1.44) | 0.320 | 0.59 (0.30–1.17) | 0.129 | 0.48 (0.14–1.57) | 0.476 | 0.95 (0.31–2.92) | 0.922 |
| Inhalational anesthetics | ||||||||
| Sevoflurane | 1 | 1 | 1 | 1 | ||||
| Isoflurane | 1.35 (0.94–1.92) | 0.102 | 1.62 (1.13–2.32) | 0.008 | 1.68 (1.02–2.76) | 0.042 | 1.85 (1.13–3.02) | 0.014 |
| Estimated blood loss | ||||||||
| <400 mL | 1 | 1 | 1 | 1 | ||||
| ≥400 mL | 1.59 (1.08–2.35) | 0.019 | 1.77 (1.20–2.59) | 0.004 | 1.89 (1.09–3.26) | 0.023 | 2.02 (1.17–3.48) | 0.011 |
| Postoperative complicationse | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.11 (0.77–1.60) | 0.578 | 1.32 (0.91–1.91) | 0.149 | 1.01 (0.61–1.65) | 0.983 | 1.27 (0.77–2.10) | 0.352 |
| Postoperative chemotherapy | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.77 (0.56–1.05) | 0.097 | 0.68 (0.49–0.95) | 0.022 | 0.76 (0.50–1.15) | 0.193 | 0.67 (0.43–1.04) | 0.074 |
Notes: aFactors with P<0.20 in univariable analyses and those that were considered clinically important were included in multivariable analyses. bIncludes dexamethasone and methylprednisolone. cIncludes intraductal papilloma, adeno-squamous carcinoma, neuroendocrine tumor, and mucinous adenocarcinoma. dAccording to the American Joint Committee on Cancer 8th Edition Cancer Staging System. eDefined as new-onset conditions that required medical intervention. Includes delayed gastric emptying, biliary leak, pancreatic leak, chylous leak, surgical site infection, atelectasis, pleural effusion, pulmonary infection, acute respiratory failure, and deep vein thrombosis.
Abbreviations: RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval. P values in bold indicate those of <0.05.
In the matched cohort, 16 factors were included in the multivariable model, including 12 factors with P<0.20 in univariable analyses (perioperative use of glucocorticoids, age ≥70 y, BMI ≥25kg/m2, preoperative hepatorenal dysfunction, ductal adenocarcinoma, higher pTNM stage, low differentiation grade, positive surgical margin, estimated blood loss ≥400 mL, type of surgery, type of anesthesia, and isoflurane inhalation) and 4 factors that were considered clinically important (preoperative CA19-9 level, period of surgery, postoperative complications, and postoperative chemotherapy). After correction for confounding factors, perioperative glucocorticoid use was significantly associated with a prolonged recurrence-free survival (HR 0.54, 95% CI 0.35–0.84, P=0.007). Among other factors, age ≥70 y (vs <60 y), preoperative hepatorenal dysfunction, ductal adenocarcinoma (vs others), higher pTNM stage (IIB, III, IV vs IA, IB, IIA), low grade differentiation (vs medium/high grade), positive surgical margin, isoflurane inhalation (vs sevoflurane inhalation), and estimated blood loss ≥400 mL were associated with shortened recurrence-free survival (Table 4; Supplement Table 7).
Discussion
In this retrospective study with propensity score-matching, 215 patients after pancreatic cancer surgery were followed up for a median of 74.0 months. In both the full cohort and the matched cohort, recurrence-free survival was significantly longer in patients with perioperative glucocorticoids than in those without; perioperative glucocorticoids were significantly associated with prolonged recurrence-free survival after correction for confounding factors.
Surgical resection is the primary treatment for patients with pancreatic cancer. However, even after radical resection and modern chemotherapy, long-term prognosis of pancreatic cancer patients remains poor. According to recent studies, the median recurrence-free survival after pancreatic cancer surgery (and chemotherapy) was 6.7–22.9 months, the median overall survival was 10.9–54.4 months, and the 5-year survival rate was 7.3–44.1%.3,4 In the present study, the median recurrence-free survival was 9.4 months (95% CI 5.1–20.3), the median overall survival was 16.8 months (95% CI 10.4–29.2), and the 5-year survival rate was 11.1% (95% CI 6.2–16.0). The results of our patients were all within the ranges of previous reports.
Glucocorticoids have long been used as the first-line therapy in patients with hematologic malignancies.30 However, the reported effects of perioperative glucocorticoids on long-term outcomes after cancer surgery vary widely and may be cancer-dependent. For example, long-term follow-up of patients after colorectal cancer surgery showed that perioperative dexamethasone was associated with an increased risk of cancer recurrence.17 Whereas studies of patients undergoing surgeries for ovarian and breast cancers did not find any significant effects of antiemetic dose dexamethasone on long-term survival.18,19 On the other hand, a retrospective study of lung cancer patients showed improved recurrence-free and overall survival with perioperative glucocorticoids.16 Studies involving pancreatic cancer patients were limited. In two retrospective studies of patients after pancreatic cancer surgery, perioperative low-dose dexamethasone (4–10 mg) was associated with the prolonged overall survival.14,15 In the present study, 52.1% of our patients received glucocorticoid (median equivalent dose of dexamethasone 10 mg) during anesthesia and/or early postoperative period. Our results also showed that, in both the full cohort and the matched cohort, perioperative glucocorticoids were associated with improved recurrence-free survival after surgery; these were in line with the above studies in pancreatic cancer patients.14,15
The potential mechanisms by which glucocorticoids may improve long-term outcomes after pancreatic cancer surgery remain unclear, but may be related to its anti-inflammatory and immunomodulatory effects. Inflammation, especially chronic inflammation, might play an important role in the occurrence and development of pancreatic cancer.5 Cytokines produced by immune and cancer cells can not only enhance cancer invasiveness but also produce immunosuppression which promote cancer cells to escape from immune surveillance.5 During the perioperative period, surgery-related inflammation is associated with profound immunosuppression, which may accelerate cancer recurrence and metastasis.31 Glucocorticoids have a broad-spectrum anti-inflammatory effect.32 In a randomized controlled trial by Kim et al,33 a single-dose dexamethasone (10 mg) significantly relieved the degree of inflammation within 24 hours after uterine arterial embolization. Along with the anti-inflammatory effect, glucocorticoids can modulate immune function by suppressing the Th1-cellular immunity axis and augmenting the Th2-mediated humoral immunity, which may provide protection against surgical stress.28,34 A study in healthy volunteers showed that the anti-inflammatory and immunomodulatory effects of a single-dose dexamethasone (8 mg) lasts for at least 24 hours.12
Concerns regarding perioperative glucocorticoid use include the possibility of increased postoperative infection and anastomotic leak, as well as elevated blood glucose which is also associated with increased postoperative complications.35,36 However, these adverse events are mainly observed in patients with prolonged glucocorticoid use,33 but not in those given only short-term therapy.37 On the contrary, a randomized controlled trial reported that perioperative hydrocortisone (100 mg every 8 hours for 3 days) reduced major complications after pancreaticoduodenectomy.38 In a recent retrospective cohort study, patients who received a single-dose dexamethasone developed less infectious complications after pancreatic cancer surgery.15 Results of the present study also showed that perioperative use of antiemetic dose glucocorticoids did not increase complications after surgery for pancreatic cancer.
It is well known that perioperative blood transfusion is associated with shortened long-term survival after cancer (including pancreatic cancer) surgery.39,40 As a matter of fact, high volume intraoperative blood loss is also associated with worse outcomes including increased postoperative complications41 and deteriorated long-term outcomes.42,43 For example, in a retrospective study of patients with stage II/III gastric cancer, intraoperative blood loss of >330 mL was associated with early recurrence after surgery.42 In a retrospective study of pancreatic cancer patients, patients with massive intraoperative blood loss had shorter overall survival and recurrence-free survival after surgery, although the associations were no longer significant after the correction for confounding factors.43 In the present study, we did not find significant associations between perioperative blood transfusion and long-term outcomes after pancreatic cancer surgery, possibly due to the small number of patients receiving blood transfusions. However, we found that high volume intraoperative blood loss (≥400 mL) was associated with shortened recurrence-free and overall survival, which is consistent with previous studies.
The impacts of inhalational anesthetics on long-term outcomes after cancer surgery attract much attention. Available studies (mainly retrospective) indicated that volatile inhalational anesthesia was associated with shortened long-term survival after cancer surgery when compared with propofol intravenous anesthesia,44 possibly due to the enhanced immunosuppression and the upregulated hypoxia-inducible-factor-1 and matrix metalloproteinases.45 However, the effects of different inhalational anesthetics might be different.45 In a study of patients undergoing cytoreductive surgery for ovarian cancer, desflurane anesthesia was associated with delayed recurrence compared with sevoflurane.46 In the present study, isoflurane anesthesia was associated with shortened overall and recurrence-free survival when compared with sevoflurane. The effects of different inhalational anesthetics on the prognosis of patients undergoing cancer surgery require further studies. Our study also found that the pathological diagnosis of ductal adenocarcinoma, high-grade TNM stage, low degree of cancer differentiation, and positive surgical margin were associated with shorter survival; whereas postoperative chemotherapy was associated with longer survival. These results are consistent with previous reports.14,15
In addition to the retrospective nature, there are other limitations of the present study. Firstly, patients included in this study underwent surgery over a period of 12 years. The chief surgeons changed during this period, and so were some routine clinical practices. For example, glucocorticoids were used more frequently and the number of surgical cases were higher in recent years. These might produce bias. In the present study, the period of surgery was used for propensity score-matching and was included in the multivariable model in order to correct for its potential confounding effects.47 Secondly, only 15 cases (7.0%) received combined epidural block during general anesthesia in our patients. This limited our ability to detect any effects of epidural block on the outcome of pancreatic cancer patients, which was suggested by previous studies.14 Lastly, as a single-center study, the generalizability of our results may be limited.
Conclusions
In summary, results of this retrospective study with propensity score-matching showed that perioperative use of low-dose glucocorticoids was associated with improved recurrence-free survival in patients undergoing radical surgery for pancreatic cancer. Considering the widespread use of glucocorticoids in the perioperative period, prospective studies are urgently needed to clarify its effect on long-term outcomes after pancreatic cancer surgery.
Acknowledgments
The authors gratefully acknowledge Dr. Bao-Cai Xing (MD, Professor, the first Department of Hepatic, Biliary & Pancreatic Surgery, Peking University Cancer Hospital, Beijing, China) for his help in data collection and Ms. Cai-Xia Yang (research assistant, the Department of Anesthesiology, Peking University Cancer Hospital, Beijing, China) for her help in postoperative follow-ups.
Funding Statement
This work was funded by the National Key R&D Program of China (2018YFC2001800). The sponsor has no role in the study design and conduct; the collection, management, analysis, and interpretation of the data; or the preparation and approval of the manuscript.
Abbreviations
TNM, tumor-node-metastasis; OS, overall survival; RFS, recurrence-free survival.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study protocol was approved by the Clinical Research Ethics Committee of Peking University Cancer Hospital (2018YJZ49).
Consent for Publication
Not applicable.
Author Contributions
DXW conceived the study and was responsible for study supervision. YXZ, DLM and DXW designed the study. YXZ and KMJ recruited patients, collected baseline and perioperative data, and performed postoperative follow-ups. YXZ, DLM, KMJ, XYL and DXW analyzed and interpreted all the data. XYL contributed to statistical analyses. YXZ drafted the manuscript. DXW and DLM critically revised the manuscript. DXW supplied administrative, technical, and material support. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dong-Xin Wang reports grants from Ministry of Science and Technology, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26. doi: 10.1038/s41571-018-0112-1 [DOI] [PubMed] [Google Scholar]
- 4.Niesen W, Hank T, Büchler M, Strobel O. Local radicality and survival outcome of pancreatic cancer surgery. Ann Gastroenterol Surg. 2019;3(5):464–475. doi: 10.1002/ags3.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci. 2019;20(3):E676. doi: 10.3390/ijms20030676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang B, Zhang LL, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122(4):1202–1210. doi: 10.1213/ANE.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 7.Duan P, Liu Y, Li J. The comparative efficacy and safety of topical non-steroidal anti-inflammatory drugs for the treatment of anterior chamber inflammation after cataract surgery: a systematic review and network meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):639–649. doi: 10.1007/s00417-017-3599-8 [DOI] [PubMed] [Google Scholar]
- 8.Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: A metaanalysis. Ochsner J. 2017;17(4):345–361. [PMC free article] [PubMed] [Google Scholar]
- 9.Forget P, Machiels JP, Coulie PG, et al. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S650–S660. doi: 10.1245/s10434-013-3136-x [DOI] [PubMed] [Google Scholar]
- 10.Zhang DF, Su X, Meng ZT, et al. Impact of dexmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-Year follow-up of a randomized controlled trial. Ann Surg. 2019;270(2):356–363. doi: 10.1097/SLA.0000000000002801 [DOI] [PubMed] [Google Scholar]
- 11.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi: 10.1213/ANE.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 12.Bain CR, Draxler DF, Taylor R, et al. The early in-vivo effects of a single anti-emetic dose of dexamethasone on innate immune cell gene expression and activation in healthy volunteers. Anaesthesia. 2018;73(8):955–966. doi: 10.1111/anae.14306 [DOI] [PubMed] [Google Scholar]
- 13.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Call TR, Pace NL, Thorup DB, et al. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology. 2015;122(2):317–324. doi: 10.1097/ALN.0000000000000489 [DOI] [PubMed] [Google Scholar]
- 15.Sandini M, Ruscic KJ, Ferrone CR, et al. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long-term survival in pancreatic cancer. Ann Surg Oncol. 2018;25(13):4020–4026. doi: 10.1245/s10434-018-6827-5 [DOI] [PubMed] [Google Scholar]
- 16.Huang WW, Zhu WZ, Mu DL, et al. Perioperative management may improve long-term survival in patients after lung cancer surgery: a retrospective cohort study. Anesth Analg. 2018;126(5):1666–1674. doi: 10.1213/ANE.0000000000002886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh PP, Lemanu DP, Taylor MH, Hill AG. Association between preoperative glucocorticoids and long-term survival and cancer recurrence after colectomy: follow-up analysis of a previous randomized controlled trial. Br J Anaesth. 2014;113(Suppl 1):i68–i73. doi: 10.1093/bja/aet577 [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Kim DW, Park S, et al. Single dose of dexamethasone is not associated with postoperative recurrence and mortality in breast cancer patients: a propensity-matched cohort study. BMC Cancer. 2019;19(1):251. doi: 10.1186/s12885-019-5451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Oliveira GS Jr, McCarthy R, Turan A, Schink JC, Fitzgerald PC, Sessler DI. Is dexamethasone associated with recurrence of ovarian cancer? Anesth Analg. 2014;118(6):1213–1218. doi: 10.1213/ANE.0b013e3182a5d656 [DOI] [PubMed] [Google Scholar]
- 20.Zhu M, Zhou C, Huang B, Ruan L, Liang R. Granisetron plus dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: A meta-analysis. J Int Med Res. 2017;45(3):904–911. doi: 10.1177/0300060517703276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opioids. in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 22.Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand. 1998;42(5):576–580. doi: 10.1111/j.1399-6576.1998.tb05169.x [DOI] [PubMed] [Google Scholar]
- 23.Downing JW, Brock-Utne JG, Barclay A, Schwegmann ILWY. 16225 (dezocine), a new synthetic opiate agonist-antagonist and potent analgesic: comparison with morphine for relief of pain after lower abdominal surgery. Br J Anaesth. 1981;53(1):59–64. doi: 10.1093/bja/53.1.59 [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Lv F, Peng M. Analgesic effect of different dosage of Flurbiprofen axetil in laparoscopic cholecystectomy in comparison with other analgesic drugs. Pak J Pharm Sci. 2017;30(5(Special)):1895–1898. [PubMed] [Google Scholar]
- 25.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63(2):200–207. doi: 10.1016/0002-9343(77)90233-9 [DOI] [PubMed] [Google Scholar]
- 26.Chouliaras K, Newman NA, Shukla M, et al. Analysis of recurrence after the resection of pancreatic neuroendocrine tumors. J Surg Oncol. 2018;118(3):416–421. doi: 10.1002/jso.25146 [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 30.Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16(6):553–563. doi: 10.1097/01.cco.0000142072.22226.09 [DOI] [PubMed] [Google Scholar]
- 31.O’Dwyer MJ, Owen HC, Torrance HD. The perioperative immune response. Curr Opin Crit Care. 2015;21(4):336–342. doi: 10.1097/MCC.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ. Glucocorticosteroids. Handb Exp Pharmacol. 2017;237:93–115. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Koo BN, Shin CS, Ban M, Han K, Kim MD. The effects of single-dose dexamethasone on inflammatory response and pain after uterine artery embolisation for symptomatic fibroids or adenomyosis: a randomised controlled study. Bjog. 2016;123(4):580–587. doi: 10.1111/1471-0528.13785 [DOI] [PubMed] [Google Scholar]
- 34.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010 [DOI] [PubMed] [Google Scholar]
- 35.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Ann Surg. 2013;257(1):8–14. doi: 10.1097/SLA.0b013e31827b6bbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slieker JC, Komen N, Mannaerts GH, et al. Long-term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left-sided colorectal anastomoses. Arch Surg. 2012;147(5):447–452. doi: 10.1001/archsurg.2011.1690 [DOI] [PubMed] [Google Scholar]
- 37.Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–248. doi: 10.1097/ALN.0000000000001466 [DOI] [PubMed] [Google Scholar]
- 38.Laaninen M, Sand J, Nordback I, Vasama K, Laukkarinen J. Perioperative hydrocortisone reduces major complications after pancreaticoduodenectomy: a randomized controlled trial. Ann Surg. 2016;264(5):696–702. doi: 10.1097/SLA.0000000000001883 [DOI] [PubMed] [Google Scholar]
- 39.Pushan Z, Manbiao C, Sulai L, Jun L, Ruidong Z, Hanshen Y. The impact of perioperative blood transfusion on survival and recurrence after radical prostatectomy for prostate cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2018;14(Supplement):S701–S707. doi: 10.4103/0973-1482.193115 [DOI] [PubMed] [Google Scholar]
- 40.Mavros MN, Xu L, Maqsood H, et al. Perioperative blood transfusion and the prognosis of pancreatic cancer surgery: systematic review and meta-analysis. Ann Surg Oncol. 2015;22(13):4382–4391. doi: 10.1245/s10434-015-4823-6 [DOI] [PubMed] [Google Scholar]
- 41.Saleh A, Ihedioha U, Babu B, Evans J, Kang P. Is estimated intra-operative blood loss a reliable predictor of surgical outcomes in laparoscopic colorectal cancer surgery? Scott Med J. 2016;61(3):167–170. doi: 10.1177/0036933015597174 [DOI] [PubMed] [Google Scholar]
- 42.Ito Y, Kanda M, Ito S, et al. Intraoperative blood loss is associated with shortened postoperative survival of patients with stage II/III gastric cancer: analysis of a multi-institutional dataset. World J Surg. 2019;43(3):870–877. doi: 10.1007/s00268-018-4834-0 [DOI] [PubMed] [Google Scholar]
- 43.Arima K, Hashimoto D, Okabe H, et al. Intraoperative blood loss is not a predictor of prognosis for pancreatic cancer. Surg Today. 2016;46(7):792–797. doi: 10.1007/s00595-015-1238-8 [DOI] [PubMed] [Google Scholar]
- 44.Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B. Global Onco-Anesthesia Research Collaboration Group. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–561. doi: 10.1007/s12630-019-01330-x [DOI] [PubMed] [Google Scholar]
- 45.Jiao B, Yang C, Huang NN, Yang N, Wei J, Xu H. Relationship between volatile anesthetics and tumor progression: unveiling the mystery. Curr Med Sci. 2018;38(6):962–967. doi: 10.1007/s11596-018-1970-6 [DOI] [PubMed] [Google Scholar]
- 46.Elias K, Kang S, Liu X, Horowitz NS, Berkowitz RS, Frendl G. Anesthetic selection and disease-free survival following optimal primary cytoreductive surgery for stage III epithelial ovarian cancer. Ann Surg Oncol. 2015;22(4):1341–1348. doi: 10.1245/s10434-014-4112-9 [DOI] [PubMed] [Google Scholar]
- 47.Catalano F, Mengardo V, Trecca A, et al. The impact of experience on short- and long-term outcomes on gastric ESD: a western series. Updates Surg. 2019;71(2):359–365. doi: 10.1007/s13304-019-00628-1 [DOI] [PubMed] [Google Scholar]