See accompanying article on page 988
The article by Ng et al1 is highlighting the presence of circulating neutrophil extracellular traps (NETs) in coronavirus disease 2019 (COVID-19) and their role as prognostic indicator. The initial description of NETs in plasma of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients in April 20202 has focused interest in neutrophil function and NET formation in this condition. Severe COVID-19 cases are putting pressure on health care systems and particularly on intensive care units with median lengths of intensive care unit stay ranging from 6 to 12 days in studies conducted in China and 4 to 19 days in studies outside of China.3 But which features make NETs determinants of clinical outcome and do we actually have sufficient data to support a specific role in COVID-19?
The concept of NETosis was introduced in 20044 as release of extracellular DNA traps by neutrophils, composed of decondensed chromatin and granule proteins. NETosis-inducing agents (Figure) are bacteria, fungi, protozoa, viruses, platelets, cytokines, and nitric oxide donors. NET formation is a form of cell death47 involving the translocation of elastase and myeloperoxidase from primary granules to the nucleus where they cleave histones after hypercitrullination catalyzed by PAD-4 (peptidylarginine deiminase 4), leading to chromatin decondensation.48 Although NET generation has been described initially as an antimicrobial mechanism, recent data suggest that NETs contribute to lung injury,30,49 vascular thrombosis,50 and multiple other conditions (Figure).
Figure.
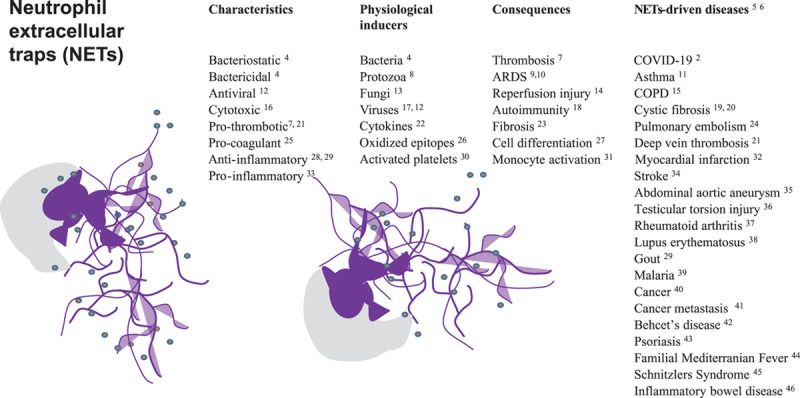
Neutrophil extracellular traps (NETs) shed from activated neutrophils (neutrophil body in gray, nucleus and NETs in purple, schematic drawing), and NETs-driven diseases. ARDS indicates acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; and COVID-19, coronavirus disease 2019.
Circulating surrogate markers of NETs in plasma are complexes of DNA and myeloperoxidase,2,9,17,51–53 citrullinated histone H3,2,51,53 cell-free DNA,2,51,53 and neutrophil elastase.51 The data of Ng1 are based on a relatively large patient number including 5-month follow-up samples compared with previous studies. However, for the assessment of outcomes, robust statistical methodology will be needed, with multivariate analyses of large sample sets corrected for confounders, such as age and cardiovascular risk factors.
Drastic changes in blood neutrophils can originate from mobilization of neutrophils from the marginated pool of the lung via CXCR4 (C-X-C motif chemokine receptor 4)–CXCL12 (C-X-C motif chemokine ligand 12) interactions leading to a spill-over of the pulmonary inflammatory process to the systemic circulation.54 Authors’ observation that circulating NETs markers correlate with markers of inflammation and endothelial damage in COVID-191 emphasize the relevance of the virus for the vasculature, and centers the causes for patients’ demise on the microvascular thrombosis aspect of the infection.50 Although SARS-CoV-2–derived mRNA may not be detectable in blood during active infection,55 the virus is able to directly infect activated neutrophils via surface ACE-2 (angiotensin-converting enzyme 2).17 Authors demonstrate derangement of the endothelial activation/damage marker VWF (von Willebrand factor) and its protease, ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motifs 13),1 which is an elegant suggestion of endothelial injury and potential microthrombosis that is in proportion with markers of NETs. Mechanisms on how virus/various-length-pieces of circulating DNA exert ADAMTS-13 suppression are unknown.
The lung retains primed neutrophils, a protective mechanism shown to be impaired in acute respiratory distress syndrome.56 NETs released by SARS-CoV-2–activated neutrophils promote lung epithelial cell death in vitro17 and neutrophil infiltration. Interstitial NETs and intravascular thrombi are characteristic features of acute respiratory distress syndrome lungs in lethal COVID-19 cases.17,57–59 NETs were found in airways together with fibrin occluding alveoli and bronchioles.57 NETs trigger coagulation60 and foster fibrin deposition in the airways compromising pulmonary ventilation and gas diffusion capacity. In accordance, Ng et al1 show that higher circulating NETs levels are associated with the need for respiratory support and with mortality, which confirms smaller studies.9 By contrast, other reports indicated that ventilator-dependent patients exhibited higher concentrations of cell-free DNA2,51 which signifies general cell death and is not specific for neutrophils.
Establishing causality between NETs burden and poor outcome highlights an urgent need for representative models of SARS-CoV-2 infection. So far, ferrets and hamsters are reported to come closest to humans, considering virus replication, clinical signs, pneumonia, transmission, immunology, and demographics.61 However, all available models to date seem to lack formation of NETs and lung thrombosis,62 suggesting that they do not serve to study severe SARS-CoV-2 infection.
Circulating deoxyribonucleases (DNase) may be another important puzzle piece in COVID-19. Deoxyribonucleases 1 and 1L3 are naturally regulating the amount of circulating extracellular chromatin, and intact endogenous plasma DNase activity is essential for homeostasis and survival.63 No data on DNase activity in patients have been published, leaving us puzzled about its association with disease severity and potential effects on circulating NETs markers. For the full picture, authors should analyze DNase activity in their samples.
Directly targeting NETs by deoxyribonucleases has been proposed as a therapeutic approach in COVID-19, even before the first data on circulating NETs markers had been published.59 Eight trials are currently registered on ClinicalTrials.gov to test the effect of NETs degradation, whereby 6 are recruiting patients with respiratory failure/acute respiratory distress syndrome. Design and outcome of these studies will likely impact our view on the role of NETs in SARS-CoV-2 pathophysiology. There are still many hurdles to take. What if there exists a significant component of immune-mediated, virus-independent immunopathology as a primary mechanism in severe disease, do NETs still play a role? Immunosenescence of neutrophils is only partially understood, but inaccurate chemotaxis and reduced pathogen clearance are expected to result in increased tissue damage.64 These observations could affect treatment success and effectiveness and might require prospective stratification of analyses. In addition, dynamics of degradation and formation of cleavage products are likely to differ between compounds and routes of administration. Inflammatory responses of monocytes to chromatin depend on fragmentation into mononucleosomes and dinucleosomes, which are histones still wrapped in DNA.31 The complexity of synergistic signaling by citrullinated nucleosomes goes beyond the cytotoxicity of circulating naked histones.31 Authors’ observation that elevated circulating NETs markers are prognostic indicators for outcomes in patients with COVID-191 is a simple and welcome puzzle piece in a tricky setting.
Sources of Funding
This research was supported by the Austrian Science Fund projects F54 and the doctoral program Cell Communication in Health and Disease W 1205-B09, and the Vienna Science and Technology Fund project LS18-090.
Disclosures
Dr Lang has relationships with drug companies, including AOPOrphan Pharmaceuticals AG, Actelion-Janssen, MSD, United Therapeutics, Medtronic, and Ferrer. In addition to being investigator in trials involving these companies, relationships include consultancy service, research grants, and membership of scientific advisory boards. The other author reports no conflicts.
Footnotes
This manuscript was sent to William C. Sessa, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 997.
References
- 1.Ng H, Havervall S, Rosell A, Aguilera K, Parv K, von Meijenfeldt FA, Lisman T, Mackman N, Thålin C, Phillipson M. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:988–994. doi: 10.1161/ATVBAHA.120.315267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair CN, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in covid-19. JCI Insight. 2020;5:e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees EM, Nightingale ES, Jafari Y, Waterlow NR, Clifford S, B Pearson CA, Group CW, Jombart T, Procter SR, Knight GM. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18:270 doi: 10.1186/s12916-020-01726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 5.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294 [DOI] [PubMed] [Google Scholar]
- 6.Döring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res. 2020;126:1228–1241. doi: 10.1161/CIRCRESAHA.120.315931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Godínez C, Carrero JC. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep. 2019;39:BSR20180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendib I, de Chaisemartin L, Granger V, Schlemmer F, Maitre B, Hüe S, Surenaud M, Beldi-Ferchiou A, Carteaux G, Razazi K, et al. Neutrophil extracellular traps are elevated in patients with pneumonia-related acute respiratory distress syndrome. Anesthesiology. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619 [DOI] [PubMed] [Google Scholar]
- 11.Granger V, Taillé C, Roach D, Letuvé S, Dupin C, Hamidi F, Noël B, Neukirch C, Aubier M, Pretolani M, et al. Circulating neutrophil and eosinophil extracellular traps are markers of severe asthma. Allergy. 2020;75:699–702. doi: 10.1111/all.14059 [DOI] [PubMed] [Google Scholar]
- 12.Schönrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front Immunol. 2016;7:366 doi: 10.3389/fimmu.2016.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urban CF, Nett JE. Neutrophil extracellular traps in fungal infection. Semin Cell Dev Biol. 2019;89:47–57. doi: 10.1016/j.semcdb.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-works with dire consequences for health. Circ Res. 2019;125:470–488. doi: 10.1161/CIRCRESAHA.119.314581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner WD, Steinbacher P, Winterberg N, Bathke AC, Klappacher M, Studnicka M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59 doi: 10.1186/s12931-015-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366 doi: 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate covid-19 pathology. J Exp Med. 2020;217:e20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dwivedi N, Radic M. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis. 2014;73:483–491. doi: 10.1136/annrheumdis-2013-203844 [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Alemán SR, Campos-García L, Palma-Nicolas JP, Hernández-Bello R, González GM, Sánchez-González A. Understanding the entanglement: Neutrophil Extracellular Traps (NETs) in cystic fibrosis. Front Cell Infect Microbiol. 2017;7:104 doi: 10.3389/fcimb.2017.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skopelja S, Hamilton BJ, Jones JD, Yang ML, Mamula M, Ashare A, Gifford AH, Rigby WF. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight. 2016;1:e88912 doi: 10.1172/jci.insight.88912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214:439–458. doi: 10.1084/jem.20160530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ząbczyk M, Natorska J, Janion-Sadowska A, Metzgier-Gumiela A, Polak M, Plens K, Janion M, Skonieczny G, Mizia-Stec K, Undas A. Prothrombotic fibrin clot properties associated with NETs formation characterize acute pulmonary embolism patients with higher mortality risk. Sci Rep. 2020;10:11433 doi: 10.1038/s41598-020-68375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki A, Skendros P, Konstantinides S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36:1405–1414. doi: 10.1093/eurheartj/ehv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awasthi D, Nagarkoti S, Kumar A, Dubey M, Singh AK, Pathak P, Chandra T, Barthwal MK, Dikshit M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic Biol Med. 2016;93:190–203. doi: 10.1016/j.freeradbiomed.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Hofbauer TM, Mangold A, Scherz T, Seidl V, Panzenböck A, Ondracek AS, Müller J, Schneider M, Binder T, Hell L, et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic Res Cardiol. 2019;114:33 doi: 10.1007/s00395-019-0740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn J, Schauer C, Czegley C, Kling L, Petru L, Schmid B, Weidner D, Reinwald C, Biermann MHC, Blunder S, et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33:1401–1414. doi: 10.1096/fj.201800752R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547 [DOI] [PubMed] [Google Scholar]
- 30.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsourouktsoglou TD, Warnatsch A, Ioannou M, Hoving D, Wang Q, Papayannopoulos V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 2020;31:107602 doi: 10.1016/j.celrep.2020.107602 [DOI] [PubMed] [Google Scholar]
- 32.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116:1182–1192. doi: 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 34.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 35.Plana E, Oto J, Medina P, Fernández-Pardo Á, Miralles M. Novel contributions of neutrophils in the pathogenesis of abdominal aortic aneurysm, the role of neutrophil extracellular traps: A systematic review. Thromb Res. 2020;194:200–208. doi: 10.1016/j.thromres.2020.07.039 [DOI] [PubMed] [Google Scholar]
- 36.Boettcher M, Meier D, Jiménez-Alcázar M, Eschenburg G, Mietzsch S, Vincent D, Klinke M, Trochimiuk M, Appl B, Tiemann B, et al. Degradation of extracellular DNA by DNase1 significantly reduces testicular damage after testicular torsion in rats. Urology. 2017;109:223.e1–223.e7. doi: 10.1016/j.urology.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 37.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40 doi: 10.1126/scitranslmed.3005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L, Bengtsson AA, Blom AM. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404 [DOI] [PubMed] [Google Scholar]
- 39.Knackstedt SL, Georgiadou A, Apel F, Abu-Abed U, Moxon CA, Cunnington AJ, Raupach B, Cunningham D, Langhorne J, Krüger R, et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci Immunol. 2019;4:eaaw0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of Neutrophil Extracellular Traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749 doi: 10.3389/fimmu.2020.01749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6 [DOI] [PubMed] [Google Scholar]
- 42.Le Joncour A, Martos R, Loyau S, Lelay N, Dossier A, Cazes A, Fouret P, Domont F, Papo T, Jandrot-Perrus M, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2019;78:1274–1282. doi: 10.1136/annrheumdis-2018-214335 [DOI] [PubMed] [Google Scholar]
- 43.Herster F, Bittner Z, Archer NK, Dickhöfer S, Eisel D, Eigenbrod T, Knorpp T, Schneiderhan-Marra N, Löffler MW, Kalbacher H, et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat Commun. 2020;11:105 doi: 10.1038/s41467-019-13756-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, Nakos K, Tsironidou V, Koffa M, Boumpas DT, et al. Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis. 2016;75:269–277. doi: 10.1136/annrheumdis-2014-205958 [DOI] [PubMed] [Google Scholar]
- 45.Bonnekoh H, Scheffel J, Wu J, Hoffmann S, Maurer M, Krause K. Skin and systemic inflammation in Schnitzler’s syndrome are associated with neutrophil extracellular trap formation. Front Immunol. 2019;10:546 doi: 10.3389/fimmu.2019.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angelidou I, Chrysanthopoulou A, Mitsios A, Arelaki S, Arampatzioglou A, Kambas K, Ritis D, Tsironidou V, Moschos I, Dalla V, et al. REDD1/Autophagy pathway is associated with neutrophil-driven IL-1β inflammatory response in active ulcerative colitis. J Immunol. 2018;200:3950–3961. doi: 10.4049/jimmunol.1701643 [DOI] [PubMed] [Google Scholar]
- 47.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925 doi: 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, Woodard W, Lezak SP, Lugogo NL, Knight JS, et al. Neutrophil extracellular traps and thrombosis in covid-19 [published online November 5, 2020]. J Thromb Thrombolysis. 20201–8. doi: 10.1007/s11239-020-02324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210:2321–2336. doi: 10.1084/jem.20130056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summers C, Singh NR, White JF, Mackenzie IM, Johnston A, Solanki C, Balan KK, Peters AM, Chilvers ER. Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax. 2014;69:623–629. doi: 10.1136/thoraxjnl-2013-204742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe covid-19. J Exp Med. 2020;217:e20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Dassler-Plenker J, Guerci P, Huynh C, Knight JS, et al. Targeting potential drivers of covid-19: neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 61.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cleary SJ, Pitchford SC, Amison RT, Carrington R, Robaina Cabrera CL, Magnen M, Looney MR, Gray E, Page CP. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br J Pharmacol. 2020;177:4851–4865. doi: 10.1111/bph.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897 [DOI] [PubMed] [Google Scholar]
- 64.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520 [DOI] [PMC free article] [PubMed] [Google Scholar]


