Figure 3.
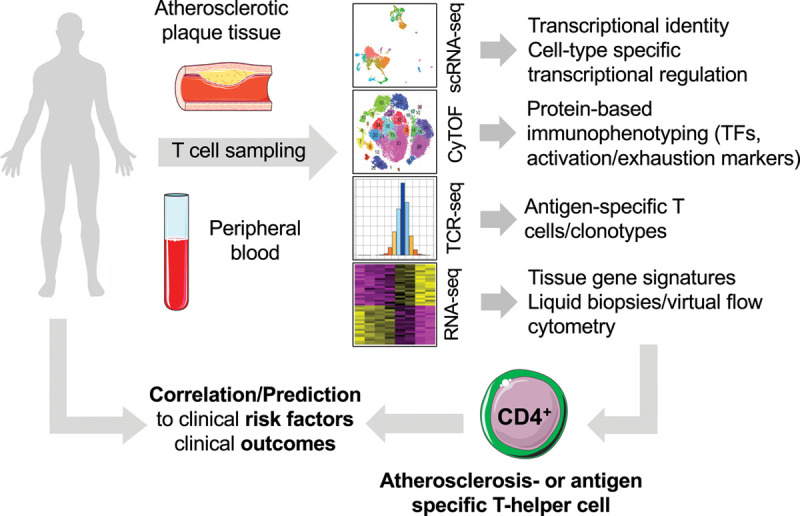
Novel high-parametric and functional immunophenotyping to develop future risk prediction tools. In humans, T cells can be routinely sampled from surgically excised carotid, peripheral arterial atherosclerotic plaques, and peripheral blood. Mass cytometry (cytometry by time of flight [CyTOF]), single cell RNA-sequencing (scRNA-seq), or a combination of antibody-staining and scRNA-seq in cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) have the potential to uncover unique, even rare, cell populations with distinct identities and potentially specialized roles in atherosclerosis (atherosclerosis-specific T cell). The addition of functional layers by staining with tetramers of MHC-II (major histocompatibility complex II) loaded with peptides from autoantigens and with T-cell receptor (TCR) sequencing in a combination with CyTOF or scRNA-seq integrates the cell surface proteome and transcriptome with antigen-specificity. Such integration generates cell type-specific gene signature, which—vice versa—are detectable in silico in bulk RNA-sequencing data sets, an approach referred to as RNA-deconvolution, liquid biopsy, or virtual flow cytometry. The correlation with clinical outcome data will be helpful to discover clinically relevant cell types. Their transcriptomes or frequencies in blood or tissue may predict the clinical outcomes or identify novel targets for future immunomodulatory cell therapy. TF indicates transcription factor. Schematics adapted from “Smart Servier Medical Art.”
