FIGURE 2.
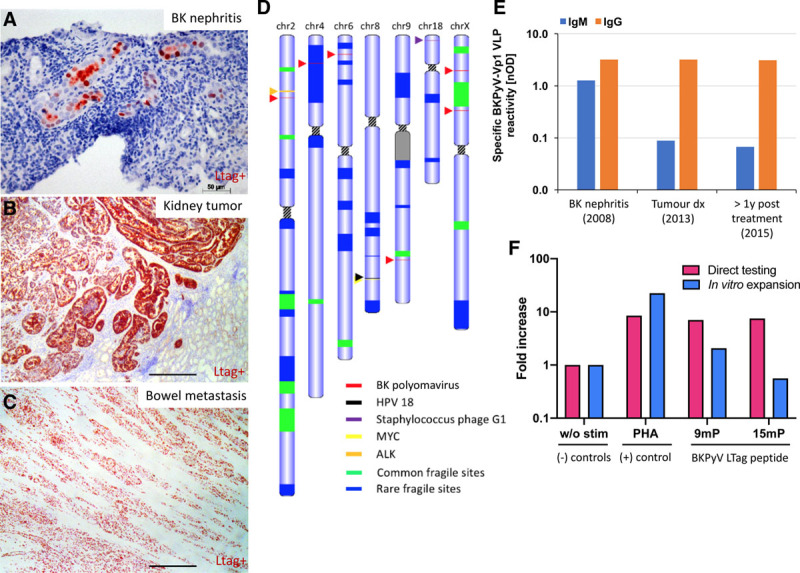
BK polyomavirus (BKPyV) integration in the collecting duct carcinoma and elicited humoral and cellular immune response. A, Large tumor antigen (LTag) staining (in red) indicating the presence of the BK virus in the kidney during the BK nephritis (before the tumor development), (B) in the kidney tumor, and (C) in the small bowel metastasis. Scale bar: 400 µm unless indicated. D, Tumor karyotype showing chromosomes with any known viruses’ integration sites and close by tumor suppressor/oncogene presence. E, Specific anti-BK virus IgM and IgG titers (against viral capsid protein 1 virus-like particles) during BK nephritis, at tumor diagnosis and 14 mo after tumor removal (labeled >1 y). F, BKPyV-specific T cell responses were quantified using interferon-γ (IFN-γ) release by enzyme-linked immunospot (ELISpot) assay using peripheral blood mononuclear cell (PBMC) collected when the patient was in remission (5 y after diagnosis). PBMCs were either stimulated directly (pink bars) or stimulated with 15mP covering the BKPyV-LTag for a 2 wk expansion in vitro before retesting (blue bars) for IFN-γ release by ELISpot. Negative control: without peptides (w/o stim); positive control: phytohemagglutinin-L (PHA-L); BKPyV peptide pools: 97 immunodominant 9mers of BKPyV EVGR (9mP) and 180 15mers overlapping by 11 amino acids spanning the entire BKPyV LTag (15mP). The results are expressed as fold increase of IFN-γ spot-forming units (SFUs) relative to negative control. PBMC response was performed in technical duplicate (direct testing) and triplicate (after in vitro expansion). ALK, anaplastic lymphoma kinase; HPV-18, human papillomavirus 18; MYC: MYC proto-oncogene; nOD, Net optical density.
