FIGURE 3.
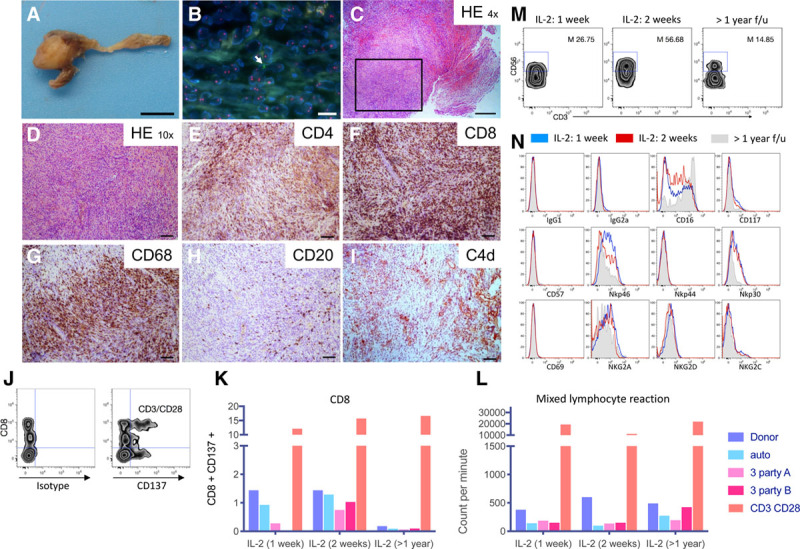
Cellular-mediated antitumor response. Macroscopic (A) and microscopic (B–I) view of the pancreatic remnant specimen retrieved around pancreatic Y graft artery 6 mo after the transplant nephrectomy. Macroscopic view scale bar: 1 cm. B, Fluorescence in situ hybridization (FISH) analysis showing rare donor cells positive for Y chromosome (arrow) and X chromosome in red. Scale bar: 10 µm. C and D, Hematoxylin and eosin staining. Scale bar: 400 and 100 µm, respectively. E–I, Immunohistochemistry staining for (E) CD4, (F) CD8, (G), CD68, (H) CD20, and (I) C4d. Scale bars: 100 µm. J, Gating strategy showing CD137 and CD8+ T cell analysis gated on patient’s live peripheral blood mononuclear cells cocultured with irradiated PE-labeled autologous cells, donor cells, two-third party stimulator cells or with CD3/CD28 beads for 36 h. K, CD8+ T cells quantification at 1 wk, 2 wk, and 14 mo after interleukin-2 (IL-2) treatment initiation (labeled >1 y). L, Mixed lymphocyte reaction of patient peripheral blood mononuclear cells cocultured with irradiated autologous cells, donor cells, third party stimulators or with CD3/CD28 beads at 1 or 2 wk after IL-2 treatment initiation and 14 mo after IL-2 treatment (performed in technical triplicates). After 6 d of culture, the proliferation was measured by thymidine incorporation and is expressed as counts per min. M, Gating strategy to define the percentage of CD3– CD56+ bright natural killer (NK) cells in the patient’s NK cell population (defined as CD3– CD56+) at 1 or 2 wk after IL-2 treatment initiation and 14 mo after IL-2 treatment (M: mean). N, Immunophenotypic analysis (IgG1 and IgG2a controls, CD16, CD57, Nkp46, NKp44, NKp30, CD69, NKG2a, NKG2d, NKg2C) of CD3– CD56+ NK cells in recipient’s peripheral blood mononuclear cells at different time points (1 wk IL-2 [gray], 2 wk IL-2 [black], or 14 mo after IL-2 treatment [shaded gray]). 7AAD+ positive dead cells were excluded of the analysis. f/u, follow-up; HE, hematoxylin eosin; NKG, NK cell group; PE, phycoerythrin.
