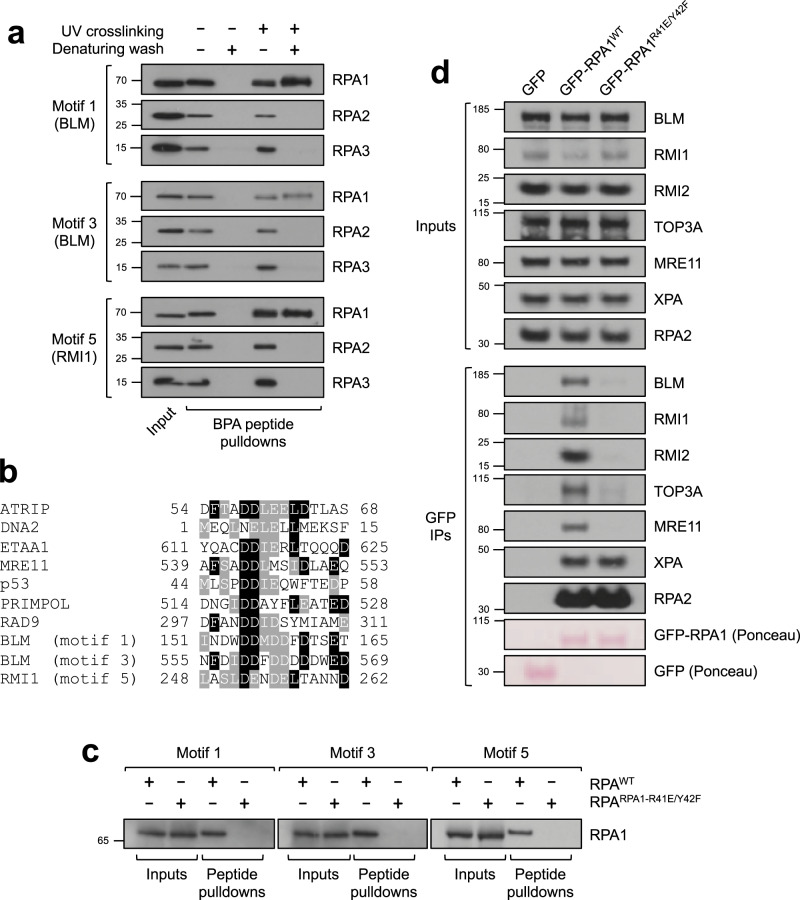
Fig. 2. The RPA-binding motifs in BLM and RMI1 interact with the RPA1 N-terminal OB-fold.
a BPA-modified peptide pulldowns from HeLa nuclear extracts with UV-crosslinking and denaturing washes where indicated, demonstrating that motifs 1, 3, and 5 interact directly with the RPA1 subunit of RPA. b Sequence alignments showing the similarity of RPA1-binding motifs in DNA damage response proteins. c Peptide pulldowns from rabbit reticulocyte lysates containing in vitro translated heterotrimeric RPA complexes incorporating either WT or R41E/Y42F RPA1, showing that all three BLM/RMI1 motifs cannot interact with the mutant RPA complex. d GFP-pulldowns from 293FT cells transfected with constructs expressing GFP or the indicated GFP-tagged RPA1 variants, showing that mutation of the N-terminal OB-fold of RPA1 (R41E/Y42F) disrupts binding to the BTR complex and MRE11. XPA is a negative control because it binds to RPA via different domains.