Abstract
Purpose
In survivors of Ebola virus disease (EVD), intraocular viral persistence raises questions about the timing and safety of cataract surgery. To the best of our knowledge, this is the first controlled study evaluating Ebola virus persistence and cataract surgery safety and outcomes in EVD survivors.
Methods
Seropositive EVD survivors and seronegative controls with vision worse than 20/40 from cataract and without active intraocular inflammation were enrolled. Aqueous humor from survivors was tested with reverse transcription–polymerase chain reaction for Ebola viral RNA. Participants underwent manual small-incision cataract surgery and 1 year of follow-up examinations.
Results
Twenty-two eyes of 22 survivors and 12 eyes of eight controls underwent cataract surgery. All of the aqueous samples tested negative for Ebola viral RNA. Median visual acuity improved from 20/200 at baseline to 20/25 at 1 year in survivors and from count fingers to 20/50 in controls (overall, P < 0.001; between groups, P = 0.07). After a 1-month course of topical corticosteroids, 55% of survivors and 67% of controls demonstrated at least 1+ anterior chamber cell. Twelve months after surgery, optical coherence tomography revealed a median increase in macular central subfield thickness of 42 µm compared with baseline (overall, P = 0.029; between groups, P = 0.995).
Conclusions
EVD survivors and controls demonstrated significant visual improvement from cataract surgery. The persistence of intraocular inflammation highlights the importance of follow-up. The absence of detectable intraocular Ebola viral RNA provides guidance regarding the safety of eye surgery in Ebola survivors.
Translational Relevance
These findings demonstrate the safety and efficacy of cataract surgery in Ebola survivors and will inform ocular surgery guidelines in this population.
Keywords: Ebola virus disease; cataract surgery; uveitis, manual small incision cataract surgery
Introduction
Ebola virus disease (EVD) is associated with a broad spectrum of systemic and ophthalmic sequelae in survivors, including uveitis, that may complicate recovery following the life-threatening illness. The Partnership for Research on Ebola Virus in Liberia (PREVAIL) III Study, a National Institutes of Health (NIH)-funded, prospective natural history study of EVD in survivors, identified uveitis in 26% of survivors and 12% of controls examined approximately 1 year after acute infection.1 Cataract, often associated with uveitis, has been identified as a reversible cause of blindness in EVD survivors.2 In the PREVAIL III study, cataract was identified in 14% of survivors and 13% of controls,1 with 28% of the cataracts qualifying as visually significant (vision worse than 20/40) in survivors compared to 20% in controls.3 Prior identification of Ebola virus persistence in ocular fluid4 resulted in hesitation to pursue cataract surgery in Ebola survivors over concern for possible transmission of the virus. Data remain limited on potential surgical risk to patients and surgical staff.2 These questions require further research and guidelines for ophthalmic surgery for EVD survivors.
From 2014 to the present, four EVD outbreaks in Africa have underscored the magnitude of this concern. The ongoing outbreak in the Democratic Republic of Congo (DRC) has affected over 3300 people, and over 1000 individuals have survived their disease.5 In Western Africa, over 28,000 people were affected by EVD between 2014 and 2016, with over 17,000 surviving.6 In 2015, as attention was directed toward EVD survivor care, the need for cataract surgery in survivors became apparent.7
Early reports of cataract surgery in EVD survivors demonstrated improvement in vision and preliminary reassurance to healthcare providers, as Ebola viral RNA was not detected in ocular fluid samples.2 However, long-term efficacy and safety data regarding cataract surgery in EVD survivors compared to controls are lacking. Given the significant EVD survivor populations who currently need cataract surgery or are expected to need it in the future, this study was undertaken.
This study, PREVAIL VII, was a collaboration among the NIH, the Liberian Ministry of Health (MOH), and other private and public partners. It prospectively explored Ebola virus ocular persistence and compared outcomes of cataract surgery between Ebola survivors and controls. Participants underwent pre- and postoperative clinical evaluation, including imaging, and were followed closely for 1 year. Aqueous samples were analyzed for evidence of Ebola virus RNA.
Methods
This was a prospective, interventional cohort study of serologically confirmed EVD survivors and close-contact controls undergoing cataract surgery in Monrovia, Liberia. It was designed and conducted by a partnership among the Liberian MOH and the National Institute of Allergy and Infectious Diseases and the National Eye Institute at the NIH, in partnership with investigators from Johns Hopkins University and Emory University. Collaborators included the L V Prasad Eye Institute's Liberia Eye Center in Monrovia, Samaritan's Purse Charities, the US Agency for International Development, and the Eternal Love Winning Africa Hospital in Monrovia. The protocol and all associated materials were approved by the National Research Ethics Board of Liberia and the Institutional Review Board of the NIH. The study abides by the tenets of the Declaration of Helsinki. All participants provided written informed consent after explanation of the nature and possible consequences of the study.
Participants
Participants were recruited from an ongoing natural history study (PREVAIL III, described elsewhere1) of EVD survivors and their close contact controls from 2014 to 2016 in Liberia. That study follows participants with periodic interviews, physical exams, and yearly detailed ophthalmologic examinations for a subset participating in an eye sub-study. The eye exams provide an opportunity to identify participants who may benefit from cataract surgery.
Participants in this study were required to be at least 14 years of age with a visually significant cataract, defined as corrected visual acuity worse than 20/40 attributable to cataract. Eyes with active inflammation, per Standardization of Uveitis Nomenclature (SUN) criteria,8 within 3 months of enrollment were excluded. Survivors were offered cataract surgery in the worse-seeing eye; surgery was not pursued in the fellow eye, given limited safety data. Controls were offered bilateral cataract surgery when indicated, with fellow eye surgery occurring approximately 1 week later, as per the standard of care in this region. EVD survivors who were not enrolled in PREVAIL III were eligible; however, no such survivors were enrolled.
Baseline and Follow-Up Examinations
Baseline ophthalmologic examination consisted of a detailed medical and ophthalmic history, visual acuity and pinhole visual acuity testing on a Tumbling-E Early Treatment Diabetic Retinopathy Study (ETDRS) vision chart, intraocular pressure measurement, and detailed slit-lamp and funduscopic exam, including ultrasound B-scan imaging when cataract prevented a posterior view, as well as optical coherence tomography (OCT) imaging of the retina and optic nerve. SUN criteria were used to grade intraocular inflammation, when present, with 1+ anterior chamber cell indicating the presence of 6 to 15 cells per 1 mm × 1 mm high-powered field (HPF), 2+ indicating 16 to 25 cells/HPF, 3+ indicating 26 to 50 cells/HPF, and 4+ indicating greater than 50 cells/HPF.
Follow-up examinations at postoperative day 1 and week 1 consisted of visual acuity and pinhole visual acuity testing, intraocular pressure, and a detailed slit-lamp examination in the operative eye, with dilated fundus examination as indicated. All other follow-up study visits (at months 1, 3, 6, 9, and 12) included interval medical and ophthalmic history, testing in both eyes of best-corrected spherical equivalent visual acuity on a Tumbling-E ETDRS vision chart, intraocular pressure, and a detailed slit-lamp and dilated funduscopic examination, as well as OCT imaging. Additional exams were performed as clinically indicated.
Surgical Procedures and Postsurgical Care
One week prior to cataract surgery, aqueous humor samples were extracted from the anterior chamber of operative eyes of seropositive participants for reverse transcription–polymerase chain reaction (RT-PCR) analysis using the GeneXpert assay (Cepheid, Sunnyvale, CA) to detect Ebola viral RNA.9 Personal protective equipment (PPE) worn by surgeons conducting the aqueous fluid collections followed Centers for Disease Control and Prevention recommendations for providers caring for patients with confirmed Ebola infection. The PPE consisted of (all disposable) impermeable gown, powered air-purifying respirator full face and neck covering with integrated blower, double-layered examination gloves with extended cuffs, and boot covers. If any eye tested positive for Ebola virus by RT-PCR, surgery was withheld.
Cataract surgery was performed using standard operating techniques for manual small-incision cataract surgery (MSICS) by two experienced eye surgeons employing standard sterile surgical precautions. A retrobulbar block was placed for anesthesia and akinesia. After aspiration of additional aqueous humor for further testing, a superior scleral tunnel incision was created. The lens cortex and nucleus were extracted and placed in formalin for future testing. A three-piece posterior chamber intraocular lens was placed in the capsular bag. In cases of posterior capsular rupture, a limited anterior vitrectomy was performed, and a three-piece intraocular lens was placed in the ciliary sulcus.
Postsurgical ocular medications consisted of topical antibiotics (ofloxacin four times a day) for 1 week and a standardized topical corticosteroid taper, beginning with prednisolone acetate 1% four times a day. Corticosteroids were adjusted as clinically indicated, with more potent and longer courses of treatment used in cases of persistent intraocular inflammation.
Statistical Methods
The analytic cohort was restricted to seropositive survivors (referred to as survivors) and seronegative close contacts (referred to as controls) who underwent cataract surgery. Seropositivity was defined as having an Ebola virus glycoprotein immunoglobulin G antibody titer of 548 enzyme-linked immunosorbent assay units (EU)/mL or above, and seronegativity was defined as having an antibody titer below 548 EU/mL. Antibody titers were measured at the PREVAIL III baseline visit. Details about the serological assays have been described previously.9
Continuous measures were summarized by medians and quartiles, and categorical variables were summarized by percentages. Best-corrected spherical equivalent visual acuity was recorded in ETDRS format. The unit of observation was an eye. Measurements taken from distinct eyes on the same individual were treated as statistically independent, given the frequently unilateral nature of uveitis or trauma. Statistical comparisons were made between operative eyes of survivors and controls, as well as between operative eyes and contralateral eyes. Multivariate linear regression models were used to test for differences in continuous measures between groups at a single time point. Logistic regression models were used to test for differences in binary outcomes. Statistical comparisons involving repeated measurements were conducted using mixed-effects linear regression models that modeled correlations between repeated measurements in the same eye and used generalized estimating equations to obtain parameter estimates. These models adjusted for age at time of enrollment and sex. Boxplots were produced to show changes in central subfield thickness over time between comparison groups. Potential outlier measurements of central subfield thickness were identified. Tests for differences in central subfield thickness were conducted twice, once with the potential outliers included and again with the potential outliers excluded.
All statistical analyses were conducted using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant.
Results
Study Participants
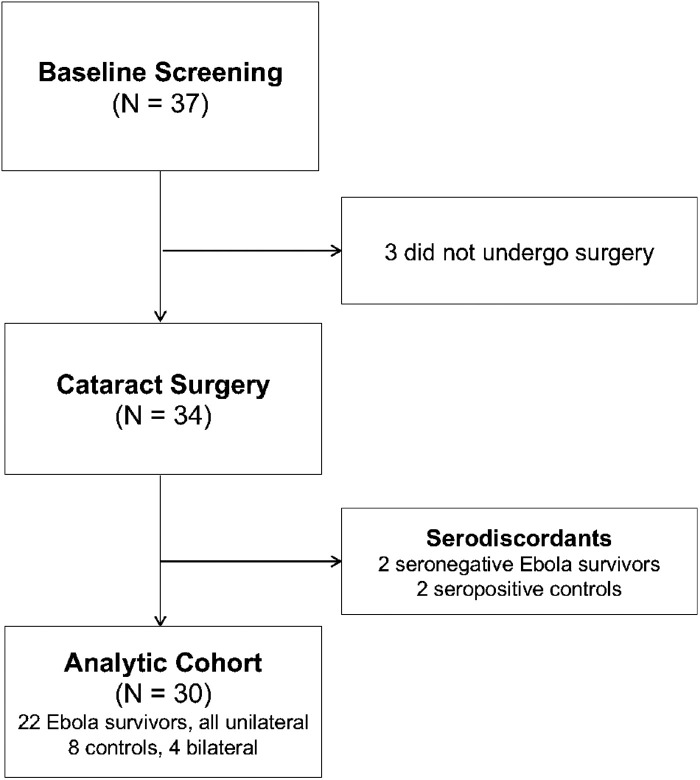
A total of 22 survivors and eight controls underwent cataract surgery. Four controls received bilateral surgery, and all survivors received unilateral surgery. Figure 1 shows a flow diagram for generation of the analytic cohort. Table 1 describes the baseline characteristics of all participants. Median age at the time of surgery was 61 years (interquartile range [IQR], 41–68) in survivors and 61 years (IQR, 50–71) in controls. Thirteen survivors (59%) and three controls (38%) were female. Median time since acute EVD infection among survivors was 36.7 months (IQR, 35.7–36.7). Evidence of uveitis—defined as the presence of at least one of the following: keratic precipitates, anterior chamber cells or flare, hypopyon, posterior synechiae, vitreous cells or haze, retinal scar (macular or peripheral), or vascular sheathing without hypertensive retinopathy—was present in 50% of survivors and 50% of controls. There were no missing data through the first 3 postoperative months. Nine percent of participants missed their 6- and 9-month postoperative visits, and one participant (3%) missed the year 1 postoperative visit due to death from hypertensive emergency.
Figure 1.
Diagram of PREVAIL VII Consolidated Standards of Reporting Trials.
Table 1.
Enrollment and Demographics of Ebola Survivors and Controls
| Ebola Survivors | Controls | Overall | |
|---|---|---|---|
| Number enrolled, n | 25 | 12 | 37 |
| Seropositive (underwent surgery) | 23 (22) | 2 (2) | 25 (24) |
| Seronegative (underwent surgery) | 2 (2) | 10 (8) | 12 (10) |
| Analytic cohorta | 22 | 8 | 30 |
| Female, n (%) | 13 (59.1) | 3 (37.5) | 16 (53.3) |
| Median (Q1, Q3) age at enrollment, y | 60.5 (40.5, 67.8) | 61 (50.2, 70.5) | 61 (42, 68.8) |
| Bilateral surgeries, n | 0 | 4 | 4 |
| History of uveitis in operative eye, n (%) | 11 (50) | 4 (50) | 15 (50) |
| Median (Q1, Q3) months since Ebola virus disease, n | 36.7 (35.7, 36.7) | NA | NA |
Seropositive Ebola survivors and seronegative controls who underwent cataract surgery.
Ocular Fluid Analysis
All serology-positive study participants underwent aqueous fluid testing for Ebola RNA by RT-PCR. All samples yielded a negative result.
Visual Acuity Outcomes
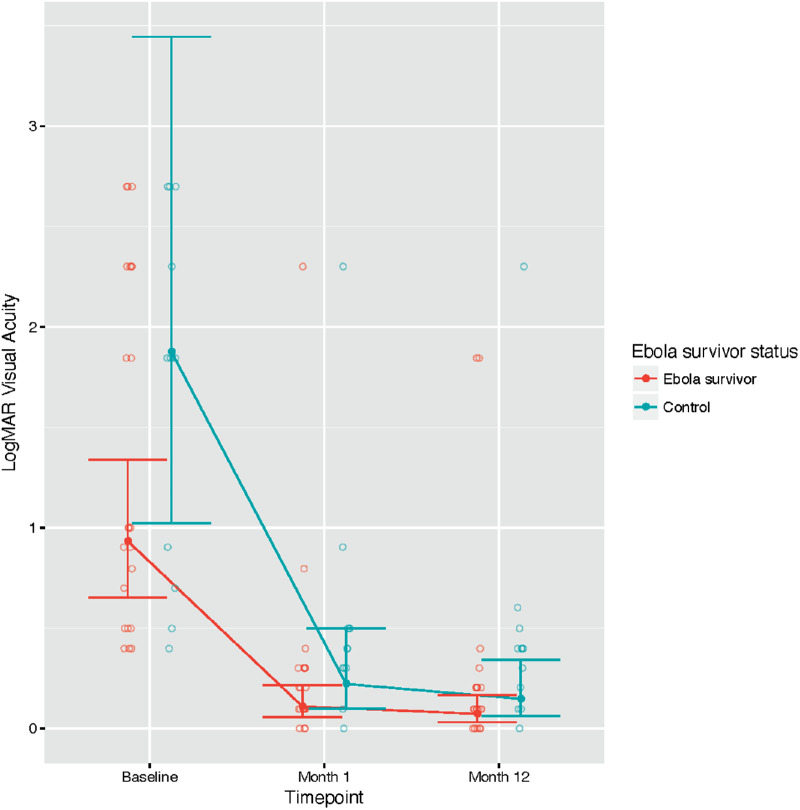
The median presurgical visual acuity at baseline was 20/200 (IQR, 20/72–20/4000) in survivors and 20/1400 (IQR 20/145–20/5500) in controls (Table 2). By 1 year after surgery, median best-corrected spherical equivalent vision improved to 20/25 (IQR, 20/25–20/32) in survivors and 20/50 (IQR 20/30–20/53) in controls; P < 0.001 for the effect of time, but there was no statistically significant difference in vision outcome between survivors and controls (P = 0.067). The median improvement in the number of lines read was 8.0 (IQR, 4.0–21.0) in survivors and 14.0 (IQR, 4.8–17.6) in controls (P = 0.465). Figure 2 shows time trends for visual acuity after surgery.
Table 2.
Visual Acuity Over Time of Ebola Survivors and Controls Following MSICS
| Ebola Survivors (n = 22 Eyes) | Controls (n = 12 Eyes) | Overall (N = 34 Eyes) | P | |
|---|---|---|---|---|
| Visual acuity (20/x), median [mean] (Q1, Q3) | Ebola survivors vs. controls, overall: P = 0.067 | |||
| Baseline | 200 [2286] (72, 4000) | 1400 [3331] (145, 5500) | 800 [2655] (100, 4000) | |
| 1 month postoperative | 28 [215] (25, 40) | 50 [384] (40, 63) | 40 [275] (25, 50) | |
| 1 year postoperative | 25 [158] (25, 32) | 50 [374] (30, 53) | 32 [237] (25, 50) | Baseline vs. year 1, overall: P < 0.001 |
| Number of lines of improvement, median [mean] (Q1, Q3) | Ebola survivors vs. controls: P < 0.465 | |||
| Baseline to 1 year postoperative | 7.96 [10.9] (4.01, 20.97) | 13.97 [12.15] (4.79, 17.55) | 8.06 [11.36] (4.01, 20.97) | |
| Spherical equivalent refractive error, mean ± SD | ||||
| 1 month postoperative | –1.39 ± 1.23 | 0.64 ± 3.46 | –0.7 ± 2.41 | |
| 1 year postoperative | –1.82 ± 1.01 | –1.67 ± 2.51 | –1.76 ± 1.75 | |
Visual acuity at 1 year relative to baseline was significantly higher across all participants (P < 0.001); the difference between survivors and controls was not significant (P = 0.067).
Figure 2.
LogMAR visual acuity over time of Ebola survivors and controls. Observed values, model estimates, and 95% CIs are shown
Postoperative Inflammation
Postoperative anterior chamber inflammation was observed in both groups (Table 3). Grade 1+ inflammation (6–15 cells/HPF) or greater was present in 55% of survivors and 67% of controls at month 1, declining to 14% in survivors and 30% in controls by month 6, with no participants showing grade 1+ or greater inflammation by month 12. There was no significant difference in inflammation between the groups (P = 0.59).
Table 3.
Summary of Anterior Chamber Cells in Operative Eyes of Ebola Survivors and Controls Following Manual Small Incision Cataract Surgery
| Anterior Chamber Cells ≥ Grade 1 (6–15 Cells/HPF), n (%) | ||||
|---|---|---|---|---|
| Month | Ebola Survivors (n = 22 Eyes) | Controls (n = 12 Eyes) | Overall (N = 34 Eyes) | P a |
| 1 | 12 (54.5) | 8 (66.7) | 20 (58.8) | |
| 3 | 11 (50) | 2 (16.7) | 13 (38.2) | |
| 6 | 3 (14.3) | 3 (30) | 6 (19.4) | 0.593 |
| 9 | 3 (15.8) | 4 (33.3) | 7 (22.6) | |
| 12 | 0 (0) | 0 (0) | 0 (0) | |
A majority of eyes demonstrated persistent inflammation at 1 month, which decreased in frequency over 1 year.
Ebola survivors versus controls.
There was no difference in postoperative inflammatory activity, as defined by the presence of anterior chamber cells or intraretinal fluid cysts by OCT, between eyes with a history of uveitis and eyes without a history of uveitis, independent of Ebola survivor status (Supplementary Table S1). Likewise, there was no difference in postoperative inflammatory activity in eyes that experienced intraoperative surgical complications compared with eyes that did not (Supplementary Table S2).
OCT Outcomes
OCT was used to identify intraretinal fluid cysts, which served as a surrogate for cystoid macular edema, and to measure macular central subfield thickness (CST). Intraretinal fluid cysts were not present in any surgical eyes preoperatively but were observed on follow-up in both survivors and controls (Table 4). They were present in 14% of survivors and 8% of controls 3 months postoperatively, decreasing to 11% of survivors and increasing to 18% of controls at 1 year. Although there was no statistically significant difference in CST when comparing survivors to controls at either baseline or follow-up visits (P = 0.995), there was a statistically significant increase in CST in both groups over time; compared to baseline, the overall increase in CST at month 12 was +42 µm (95% confidence interval [CI], +4 to +79; P = 0.029). When excluding eyes with discrete intraretinal fluid cysts, CST still increased postoperatively in both groups but lost statistical significance (+21 µm; 95% CI, –8 to +50; P = 0.148).
Table 4.
Longitudinal Summary of CST in Operative Eyes of Ebola Survivors and Controls Following MSICS
| Ebola Survivors (n = 22 Eyes) | Controls (n = 12 Eyes) | Overall (N = 34 Eyes) | Comments | |
|---|---|---|---|---|
| Intraretinal fluid cysts, n (%) | Ebola survivors vs. controls, overall: P = 0.832 | |||
| Baseline | 0 (0) | 0 (0) | 0 (0) | |
| Month 1 | 0 (0) | 0 (0) | 0 (0) | |
| Month 3 | 3 (14.3) | 1 (8.3) | 4 (12.1) | |
| Month 6 | 1 (4.8) | 0 (0) | 1 (3.3) | |
| Month 9 | 2 (10.5) | 1 (8.3) | 3 (9.7) | |
| Month 12 | 2 (10.5) | 2 (18.2) | 4 (13.3) | |
| CST median (Q1, Q3), µm | ||||
| Baseline | 209 (184, 244.5) | 203 (182, 204) | 203.5 (180.5, 228.5) | Baseline vs. follow-up: P = 0.029 |
| Month 1 | 224 (211, 269.5) | 222 (217.5, 260) | 223 (211, 269) | Ebola survivors vs. controls, overall: P = 0.995 |
| Month 3 | 216 (188, 253) | 227 (196.8, 260.2) | 220 (196, 253) | |
| Month 6 | 234 (203, 258) | 226.5 (209.5, 234.8) | 233 (207, 253.5) | CST change from baseline to follow-up: 42 (95% CI, 4–79) |
| Month 9 | 238 (214, 254.5) | 240 (227.8, 258.2) | 240 (217.5, 256) | |
| Month 12 | 231 (212.5, 245.5) | 244 (221.5, 264.5) | 238.5 (212.2, 247.8) | Month 1 vs. month 12: P = 0.352 |
| CST (excluding cases of intraretinal fluid cysts), µm | ||||
| Baseline | 218 (190.2, 244) | 203 (182, 204) | 204 (182, 227) | Baseline vs. follow-up: P = 0.148 |
| Month 1 | 224 (211, 269.5) | 222 (217.5, 260) | 223 (211, 269) | Ebola survivors vs. controls, overall: P = 0.441 |
| Month 3 | 214 (183.5, 224) | 223 (196.5, 248) | 215 (188, 233) | |
| Month 6 | 229 (202.8, 251.2) | 220 (207, 234) | 224 (207, 245) | |
| Month 9 | 238 (211, 254) | 240 (225.5, 253) | 239 (216.2, 254.2) | CST change from baseline to follow-up: 21 (95% CI, –8 to 50) |
| Month 12 | 231 (216, 244) | 242 (211, 248) | 234.5 (212.2, 246.2) | Month 1 vs. month 12: P = 0.696 |
OCT of eyes before and after cataract surgery revealed an increase in central subfield thickness during follow-up visits relative to baseline measurements (P = 0.029). The difference between baseline and follow-up was not significant when excluding cases of cystoid macular edema (P = 0.148). Macular thickening did not appear to worsen between months 1 and 12 (P = 0.352).
Complications and Postoperative Course
Surgery in both groups was well tolerated, with no statistically significant differences between survivors and controls in rates of intraoperative or postoperative complications. There were no complications resulting from the anterior chamber taps. Median duration of cataract surgery was 15.5 minutes (IQR, 14.2–20) for survivors and 19 minutes (IQR, 18–39.8) for controls (P = 0.004) (Table 5). Three survivors (14%) and five controls (42%) experienced intraoperative surgical complications (P = 0.085) (Table 5). Posterior capsule rupture occurred in one survivor and two controls, and zonular dehiscence occurred in one survivor with a history of orbital trauma. The other complications included (in one participant each): anterior capsular radial tear, iris sphincter tear, rupture of pre-existing posterior capsular scar, and small inferior tear in Descemet's membrane (Table 5).
Table 5.
Summary of Intraoperative Complications and Duration of Surgery in Operative Eyes of Ebola Survivors and Controls
| Ebola Survivors (n = 22 Eyes) | Controls (n = 12 Eyes) | Overall (N = 34 Eyes) | P | |
|---|---|---|---|---|
| Complications, n (%) | Ebola survivors vs. controls: P = 0.085 | |||
| Posterior capsule rupture(with vitreous loss) | 0 (0) | 2 (16.7) | 2 (5.9) | |
| Posterior capsule rupture(without vitreous loss) | 1 (4.5) | 0 (0) | 1 (2.9) | |
| Zonular dehiscence(without vitreous loss) | 1 (4.5) | 0 (0) | 1 (2.9) | |
| Anterior capsular radial tear | 1 (4.5) | 0 (0) | 1 (2.9) | |
| Iris sphincter tear | 0 (0) | 1 (8.3) | 1 (2.9) | |
| Rupture of pre-existingposterior capsular scar | 0 (0) | 1 (8.3) | 1 (2.9) | |
| Small inferior tear inDescemet's membrane | 0 (0) | 1 (8.3) | 1 (2.9) | |
| Any complication | 3 (13.6) | 5 (41.7) | 8 (23.5) | |
| Duration of surgery (min),median (Q1, Q3) | 15.5 (14.2, 20) | 19 (18, 39.8) | 18 (15, 23.8) | Ebola survivor vs. control: P = 0.004 |
There were no cases of early procedure termination, iridodialysis, iris prolapse, hyphema, zonular dehiscence with vitreous loss, dropped lens nucleus, choroidal hemorrhage, aqueous misdirection, or any other noted complication.
Postsurgical complications are summarized in Table 6. One survivor and one control developed subluxed intraocular lenses, observed at postoperative months 2 and 1, respectively. The control participant developed an asymptomatic retinal detachment discovered at postoperative month 12, prompting surgical repair. Thirty-six percent of survivors and 25% of controls presented with elevated intraocular pressure (IOP) greater than 21 mmHg on at least one postoperative visit (P = 0.391), compared with 0% and 17% (n = 2) at baseline in survivors and controls, respectively. Neither of the two controls who had elevated IOP at baseline had elevated IOP during any of the follow-up visits. By 1 year after cataract surgery, 32% of survivors and 8% of controls required yttrium–aluminum–garnet (YAG) capsulotomy for visually significant posterior capsule opacification (P = 0.349). There were no cases of postoperative endophthalmitis.
Table 6.
Summary of Postoperative Complications in Eyes of Ebola Survivors and Controls Following Cataract Surgery
| Postoperative Complications, n (%) | Ebola Survivors (n = 22 Eyes) | Controls (n = 12 Eyes) | Overall (N = 34 Eyes) | P a |
|---|---|---|---|---|
| Subluxed intraocular lens | 1 (4.5) | 1 (8.3) | 2 (5.9) | |
| Retinal detachment | 0 (0) | 1 (8.3) | 1 (2.9) | |
| IOP ≥ 21 at any postoperative visit | 8 (36.4) | 3 (25) | 11 (32.4) | 0.391 |
| YAG capsulotomy | 7 (31.8) | 1 (8.3) | 8 (23.5) | 0.349 |
There were no cases of postoperative endophthalmitis. Subluxed intraocular lens was observed in one Ebola survivor at postoperative month 2 and one control at postoperative month 1. Inferior rhegmatogenous retinal detachment was discovered incidentally at postoperative month 12, occurring in the same control who had a subluxed intraocular lens.
Survivors versus controls.
Discussion
To the best of our knowledge, this is the first prospective study to report the visual acuity and OCT outcomes of cataract surgery in the eyes of EVD survivors, compared to controls, with 1 year of close follow-up after surgery. The results reveal significant improvement in visual acuity in both survivors and controls, with median improvements of 8 and 14 lines of vision, respectively. The majority of survivors presented with visual acuity of 20/200 or poorer but improved to 20/40 or better by 1 year following surgery.
This study, in combination with other reports to date of cataract surgery in EVD survivors, offers additional reassurance that MSICS performed on Ebola survivors who are many months past their acute infection and have no active inflammation at the time of surgery can be safely performed for both the patient and the surgeon. In this study, aqueous humor aspirated from eyes of EVD survivors 1 week prior to cataract surgery did not reveal evidence of Ebola viral RNA by RT-PCR, consistent with findings from the Ebola Virus Persistence in Ocular Tissues and Fluids cataract study of 34 EVD survivors in Sierra Leone.2
Although the combined numbers from all reported cataract surgeries in EVD survivors are limited, these collective findings may temper the absolute need for aqueous taps when planning cataract surgery in eyes of Ebola survivors who are without active uveitis. The precise timing of Ebola virus RNA clearance is unknown; one prior study of a patient with documented ocular viral persistence showed no evidence of Ebola viral RNA in the ocular fluid by 12 months.10 Our study also confirms that, when the eye is without intraocular inflammation in an individual who is many months past EVD infection (median of 37 months in our cohort), there appears to be no active intraocular viral persistence. This is particularly important given the currently limited capacity to perform RT-PCR laboratory testing in the countries that have been affected by Ebola epidemics. In addition, it is reasonable to expect that the surgical cataract burden of Ebola survivors from the DRC and West Africa will continue to increase. Further studies of viral persistence in the eyes of Ebola survivors are warranted. In addition, development of assays tailored to assessment of eye tissues and fluids should be encouraged, as the GeneXpert and other assays used in other ocular Ebola studies were designed to analyze different body fluids and, though validated, should be standardized for this purpose.
Intraoperative complications occurred at similar rates between survivors and controls. These rates, higher than those reported in high-volume surgical centers using MSICS11,12 and phacoemulsification12 in India, highlight the importance of population-specific data. Factors that may have contributed to an increased rate of intraoperative complications in this cohort include a history of uveitis in half of operative eyes and history of eye trauma in some participants. We attribute the longer median surgical time in controls (by 3.5 minutes) compared with survivors to the possible greater complexity of cataract surgery in that group; the median presenting visual acuity was 20/1400 in controls versus 20/200 in survivors.
Postoperative complications occurred with similar rates between survivors and controls, comparable to those reported in high-volume surgical centers using MSICS.12 A significant proportion of survivors and controls exhibited persistent intraocular inflammation beyond the 1-month postoperative visit, necessitating increased frequency, potency, or duration of topical corticosteroid therapy. Involvement of the control group indicates that this may be a feature of MSICS in this setting, rather than a finding specific to survivors of EVD.
The significance of increased macular CST during follow-up compared with baseline among survivors and controls is unclear but suggests subclinical macular edema. We explored possible factors that may have predisposed a patient to an increase in CST such as history of uveitis or intraoperative surgical complication and found no difference based on these criteria. Persistence of increased macular thickness has been reported following MSICS for 6 months13 and following phacoemulsification for 2 months.14,15 Further research could explore the potential benefit of prophylactic preoperative corticosteroids and/or postoperative nonsteroidal anti-inflammatory drugs to reduce both ocular inflammation and macular thickening, which may be a response to low-grade inflammation.
One limitation of this study is the small cohort, resulting in underpowered comparisons between groups and greater variability in estimates. This could partly explain the 50% rate of prior uveitis in the control group, compared with a rate of 12% in the PREVAIL III control group from which this cohort was drawn. It is also possible that those with greatest visual impairment elected to participate in the study, with that group possibly enriched for a history of uveitis. Other causes of uveitis in both the Ebola survivor and control groups likely included toxoplasmosis, syphilis, and sarcoidosis, but further analysis was outside the scope of this study. A dedicated population study to better understand causes of uveitis within this population would be helpful. Regarding visual acuity assessments, outreach facilities, where some recruiting was performed, were not equipped with phoropters; therefore, pinhole visual acuity was used to achieve consistency and address refractive error across all participants at enrollment.
This study offers insight into planning a surgical program in the setting of an emerging infectious disease in a resource-limited setting. Coordination and collaboration across organizations were crucial for patient recruitment, facilitating operative procedures, and providing extended postoperative follow-up care. Equipment needs consisted of basic ophthalmic diagnostic instrumentation, including ultrasound B-scan at the screening visit, portable anterior vitrector during surgery, and YAG laser postoperatively. Infection prevention and control included appropriate PPE. Minimal postoperative medication needs included topical antibiotics, topical corticosteroids, and ocular hypotensive agents. Endophthalmitis treatment kits were available, although none was needed.
In summary, we report a controlled, prospective study of cataract surgery outcomes in EVD survivors, who remain at risk for ophthalmic sequelae and vision disability from uveitis and cataract. The potential for Ebola virus persistence within the eye mandates improved understanding of this condition. This work will contribute to the body of evidence that will inform safety guidelines for intraocular surgery in Ebola survivors.
Supplementary Material
Acknowledgments
The authors thank Beth Thompson and Karen Daniels of Samaritan's Purse International Relief for surgical planning and onsite surgical logistics and operations, and we thank Samaritan's Purse for provision of surgical equipment and supplies. We thank Eternal Love Winning Africa Hospital and staff in Monrovia, Liberia, for support to surgical operations and providing accommodations to patients. We thank the L V Prasad Eye Institute in Hyderabad, India and L V Prasad Eye Institute's Liberian Eye Center in Monrovia, Liberia, for help with providing postoperative care to patients. We thank the Liberian Institute for Biomedical Research laboratory in Monrovia, Liberia, for sample processing. We thank Jeff Sanderson of John Snow Incorporated for help with project coordination. We thank the US Agency for International Development for support through the Ebola Transmission Prevention and Survivor Services Program. We thank Catherine Gargu, Yassah Sosu, Jennie Sackor, Precious Cooper, Augustine Wallace, Ruth Nyain, Famatta Kamara, James Mulbah, Mercy Bowulo, Zipporah Cheburet, Dennis Kipkorir, Emily Chepkoech, Robert Koskey, John Troke, Alison Jordan, Rick Wood, Bev Kauffeldt, and Emmanuel Nyah for help caring for and communicating with study participants. Finally, we thank the Social Mobilization Team for coordinating participant visits.
Supported in part by the Division of Intramural Research of the National Institute of Allergy and Infectious Disease (NIAID) and the National Eye Institute (NEI) of the National Institutes of Health; by the NEI (award numbers K23 EY030158, JGS; RO1 EY029594, SY); by the National Cancer Institute (Contract No. HHSN261200800001E, IC); and by an American Society of Cataract and Refractive Surgery Foundation grant (“Cataract Surgical Outcomes Among Survivors of Ebola Virus Disease,” AOE).
Supported by an unrestricted departmental grant from Research to Prevent Blindness to the Emory Eye Center, Emory University School of Medicine. NIAID and NEI participated in the design of the study and conducting the study. Other sponsors and funding organizations had no role in the design or conduct of this research.
Disclosure: A.O. Eghrari, None; J.G. Shantha, None; R.D. Ross, None; C. Van Ryn, None; I. Crozier, None; B. Hayek, None; D. Gradin, None; B. Roberts, None; S.G. Prakalapakorn, None; F. Amegashie, None; K. Nishant, None; G. Singh, None; R. Dolo, None; J. Fankhauser, None; B. Burkholder, None; J. Pettitt, None; R. Gross, None; T. Brady, None; B. Dighero-Kemp, None; C. Reilly, None; L. Hensley, None; E. Higgs, None; S. Yeh, None; R.J. Bishop, None
References
- 1.PREVAIL III Study Group, Sneller MC, Reilly C, et al.. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019; 380(10): 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shantha JG, Mattia JG, Goba A, et al.. Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) Study: reverse transcription-polymerase chain reaction and cataract surgery outcomes of Ebola survivors in Sierra Leone. EBioMedicine. 2018; 30: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eghrari AO, Bishop RJ, Ross RD, et al.. Characterization of Ebola virus-associated eye disease. JAMA Network Open. 2021; 4(1): e2032216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varkey JB, Shantha JG, Crozier I, et al.. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med; 2015; 372(25): 2423–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aruna A, Mbala P, Minikulu L, et al.. Ebola virus disease outbreak – Democratic Republic of the Congo, August 2018 – November 2019. MMWR Morb Mortal Wkly Rep. 2019; 20; 68(50): 1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. 2014-2016 Ebola Outbreak in West Africa. Available at: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html. Accessed May 6, 2020.
- 7. Bishop RJ, Eghrari AO. Eye disease in EVD survivors in Liberia (PREVAIL III Study). In: WHO Meeting on Survivors of Ebola Virus Disease: Clinical Care of Survivors, Meeting Report. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 8. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140(3): 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettitt J, Fallah M, Nason M, et al.. Assessment and optimization of the GeneXpert diagnostic platform for detection of Ebola virus RNA in seminal fluid. J Infect Dis. 2017; 215(4): 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shantha JG, Crozier I, Varkey JB, et al.. Long-term management of panuveitis and iris heterochromia in an Ebola survivor. Ophthalmology. 2016; 123(12): 2626–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haripriya A, Chang DF, Reena M, et al.. Complication rates of phacoemulsification and manual small-incision cataract surgery at Aravind Eye Hospital. J Cataract Refract Surg. 2012; 38(8): 1360–1369. [DOI] [PubMed] [Google Scholar]
- 12. Venkatesh R, Muralikrishnan R, Balent LC, et al.. Outcomes of high volume cataract surgeries in a developing country. Br J Ophthalmol. 2005; 89(9): 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghosh S, Roy I, Biswas P, et al.. Prospective randomized comparative study of macular thickness following phacoemulsification and manual small incision cataract surgery. Acta Ophthalmol. 2010; 88(4): e102–e106. [DOI] [PubMed] [Google Scholar]
- 14. Mentes J, Erakgun T, Afrashi F, et al.. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. 2003; 217(6): 408–412. [DOI] [PubMed] [Google Scholar]
- 15. Biro Z, Balla Z, Kovacs B.. Change of foveal and perifoveal thickness measured by OCT after phacoemulsification and IOL implantation. Eye. 2008; 22(1): 8–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.