Abstract
Purpose
To evaluate the role of CD4+ T helper cells in benzalkonium chloride (BAC)-induced ocular surface disorder in C57BL/6 mice.
Methods
Topical 0.075% BAC was applied twice daily in C57BL/6 mice for 7 consecutive days; PBS-treated and untreated mice served as controls. Adoptive transfer of CD4+ T cells isolated from the BAC-treated mice or PBS-treated mice into nude mice was conducted to identify the roles of CD4+ T cells, with untreated nude mice as controls. Oregon green dextran staining, PAS staining, and the phenol red cotton test were carried out in these two models. The gene and protein levels of T-bet, IFN-γ, RORγt, and IL-17 were detected by quantitative RT-PCR and ELISA, respectively. The activation and subsets of CD4+ T cells were identified by double immunofluorescent staining and flow cytometry.
Results
An increase in CD4+CD69+, CD4+IFN-γ+, and CD4+IL-17+ cells was induced by BAC in C57BL/6 mice. IFN-γ, IL-17, Th1, Th17, and the transcription factors T-bet and RORγt were increased in BAC-treated mice compared with control mice. In addition, ocular surface damage, including corneal barrier dysfunction, goblet cell loss, and decreased tear production, was induced by BAC. Interestingly, adoptive transfer of CD4+ T cells isolated from BAC-treated mice into nude mice resulted in ocular surface manifestations similar to those of direct topical BAC treatment of C57BL/6 mice, including increased CD4+ T cells, IFN-γ, IL-17, and ocular surface disorders.
Conclusions
Topical application of BAC induced a dry-eye-like ocular surface disorder partly through the CD4+ T cell-mediated inflammatory response.
Keywords: benzalkonium chloride, CD4+ T cell, T-bet, RORγt
Benzalkonium chloride (BAC) is commonly used as a preservative in ophthalmic solutions due to its antimicrobial activities.1,2 Studies have demonstrated, though, that long-term use of topical BAC induces toxic effects, including tear film instability, corneal barrier dysfunction, and the loss of conjunctival goblet cells, all of which cause dry-eye-like ocular surface damage,1,3,4 However, the precise mechanism must be further explored.
The conjunctiva is a continuous membrane that serves as the junction between the eyeball and the eyelids. The conjunctiva is also a mucosa-associated lymphoid tissue.5 There are many immune cells in the normal conjunctiva, such as T helper cells and macrophages.6 When exogenous antigens initially contact mucosal membranes, they encounter potent innate and adaptive immune surveillance mechanisms.7 Immune cells in conjunctiva serve as a barrier against infection and exogenous material; however, immune responses may cause ocular surface damage and result in immune-mediated ocular surface disorders, including dry eye.8
CD4+ T cells are residents of the ocular surface and include Th1, Th2, Th17, and Treg cells. Activated CD4+ T cells release inflammatory cytokines, leading to ocular surface damage; for example, IFN-γ released by Th1 cells causes epithelial cell apoptosis, squamous metaplasia, and loss of goblet cells,9–11 and IL-17 released by Th17 cells causes corneal barrier dysfunction.12 Although the immunopathogenesis of the ocular surface immune response remains largely unknown, growing evidence indicates that excessive ocular microenvironmental stress promotes the activation of autoreactive lymphocytes.13,14
There is some evidence indicating that BAC induces tear film instability and tear film breakup time,15,16 which induces inflammation and initiates the immune response.6 Galletti's group17 has shown a loss of immune tolerance after exposure to BAC. Few studies have focused on the association between the toxicity of BAC and the immune response. Our previous research demonstrated that topical administration of BAC increased CD4+ T cells infiltration in the ocular surface,18 but the role of CD4+ T cells in BAC treatment of the ocular surface remains unknown.
In the present study, we further investigated the association between BAC toxicity and the immune response by focusing on the role of CD4+ T cells and the CD4+ T cell-mediated inflammatory response in C57BL/6 mice.
Materials and Methods
Animals
Female C57BL/6 mice and nude mice, 10 to 12 weeks old (Shanghai SLAC Laboratory Animal Center, Shanghai, China), were used in this study. All mice were kept in a standardized environment as follows: a constant temperature of 25° ± 1°C, a relative humidity of 60% ± 10%, and alternating 12-hour light/dark cycles (8:00 AM to 8:00 PM). All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and animal experimental procedures were approved by the Animal Ethics Committee of Xiamen University School of Medicine (approval ID XMUMC: 2017-2-10).
BAC Treatment
C57BL/6 mice were randomly divided into a BAC treatment group, a PBS treatment group, and an untreated (UT) group. The BAC treatment group was topically treated with 5 µL of 0.075% BAC (Sigma-Aldrich, St. Louis, MO, USA) in PBS, and the PBS-treated and UT group served as controls. The mice received BAC or PBS treatment twice daily (9:00 AM and 9:00 PM) for 7 consecutive days. The Schirmer test was performed in all groups on day 7. On day 7, all mice were sacrificed, and the eyes and adnexa were dissected for histological analysis (n = 5) and inflammatory factor detection (n = 5).
CD4+ T Cell Isolation and Adoptive Transfer Experiment
To evaluate the role of pathogenic CD4+ T cells, adoptive transfer experiments were performed as previously described.11,19 Briefly, CD4+ T cells in the spleen and cervical lymph nodes (CLNs) were collected from the BAC-treated and PBS-treated mice by negative selection using rat anti-mouse CD4-conjugated magnetic microbeads (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were washed with PBS before being used for adoptive transfer experiments. A total of 5 × 106 CD4+ T cells were transferred intraperitoneally into nude mice. The nude mice were divided into three groups: (1) BAC-treated nude mice that received CD4+ T cells isolated from BAC-treated C57BL/6 mice, (2) PBS-treated nude mice that received CD4+ T cells isolated from PBS-treated C57BL/6 mice, and (3) UT nude mice, which served as a control group. At 5 days after adoptive transfer, all nude mice were sacrificed.
Measurement of Tear Volume
The volume of tear secretion was measured using Zone-Quick phenol red cotton threads (Yokota, Tokyo, Japan) before the mice were sacrificed at the same time (7:00 PM) in the standard environment. One millimeter of phenol red thread was placed on the conjunctival sac at approximately one-third of the lower eyelid from the lateral canthus. Each eye was tested for 15 seconds. The red part of the thread was measured in millimeters, and the average length was considered the final length.
Corneal Oregon Green Dextran Staining
Invitrogen Oregon Green dextran (OGD, 70,000 MW, catalog no. D7172; Thermo Fisher Scientific, Waltham, MA, USA) staining was conducted in all groups as reported previously to assess the corneal epithelial barrier.12 Topical application of 1 µL OGD (50 mg/mL) was performed on the murine ocular surface for 1 minute, and then the corneas were washed five times with 1 mL PBS. The corneas were observed with a stereoscopic zoom microscope (AZ100; Nikon, Tokyo, Japan) under fluorescence excitation at 470 nm. The mean fluorescence intensity of OGD-positive staining in the central area of the cornea with a diameter of 2 mm was measured and analyzed using NIS-Elements 4.1 software (Nikon USA, Melville, NY, USA).
Measurement of Goblet Cell Density
The ocular tissues of the mice were dissected. The right eyeballs were embedded in paraffin. PAS reagent (catalog no. 395B-1KT; Sigma-Aldrich) was used to stain goblet cells. Digital images of paraffin sections were captured with a light microscope (Eclipse 50i; Nikon). The number of goblet cells was counted in the superior and inferior conjunctiva using NIS-Elements software.
Immunofluorescence Staining
The left eyeballs were embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, catalog no. 4583; Sakura Finetek USA, Torrance, CA, USA). Frozen sections (5 µm thick) were fixed with acetone for 15 minutes at –20°C. Subsequently, the samples were washed three times with PBS, followed by blocking with 2% BSA in PBS for 1 hour at room temperature. The samples were then incubated with monoclonal rabbit anti-CD4 antibody (1:200, 3.475 µg/mL, catalog no. ab183685; Abcam, Inc., Cambridge, MA, USA) and counterstained with polyclonal rabbit anti-IL-17 antibody (1:200, 5 µg/mL, catalog no. ab79056; Abcam) and monoclonal mouse anti-IFN-γ antibody (1:50, 4 µg/mL, catalog no. sc-12755; Santa Cruz Biotechnology, Dallas, TX, USA) diluted with 1% BSA overnight at 4°C. After they were washed three times with PBS, the samples were incubated in secondary antibody (goat-anti-rabbit IgG conjugated with Alexa Fluor 488, goat-anti-rabbit IgG conjugated with Alexa Fluor 594, or goat-anti-rat IgG conjugated with Alexa Fluor 594; Thermo Fisher Scientific) diluted with 1% BSA for 1 hour at room temperature. Finally samples were counterstained with 4′,6-diamidino-2-phenylendole (DAPI, catalog no. H-1200; Vector Laboratories, Inc., Burlingame, CA, USA). The results were photographed with a Leica upright microscope (DM2500; Leica Microsystems, Wetzlar, Germany).
Immunohistochemistry
Cryosections were immunostained for CD4 using primary rat anti-mouse CD4 antibody (1:100, 5 µg/mL, catalog no. 553647; BD Biosciences Pharmingen, San Diego, CA, USA) for 60 minutes, goat anti-rat antibody (1:50, 10 µg/mL, catalog no. 559286; BD Biosciences Pharmingen) diluted in 1% BSA for 30 minutes, and Vectasta VECTASTAIN Elite ABC system using NovaRED reagent (catalog no. PK-6100; Vector Laboratories). The sections (three slides per animal) were photographed using a Nikon Eclipse 50i digital camera. The total number of CD4+ T cells in the conjunctiva was counted using NIS-Elements software.
Paraffin sections were immunostained with primary rabbit anti-mouse T-bet (1:500, 1 µg/mL, catalog no. PA540573; Thermo Fisher Scientific) or rabbit anti-mouse RORγt (1:3000, 0.209 µg/mL, catalog no. ab207082; Abcam) antibodies as previously described.20 The sections (three slides per animal) were photographed using a Nikon Eclipse 50i digital camera.
ELISA
One sample consisted of the pooled conjunctiva of both eyes or CLNs of one animal. Protein was extracted with cold radioimmunoprecipitation assay buffer (catalog no. R0278; Sigma-Aldrich). The Pierce BCA Protein Assay Kit (catalog no. 23225; Thermo Fisher Scientific) was used to measure the total concentration of protein. The protein in the same tissue was diluted to the same concentration, and 20 µg of each sample was added to the sample well. The levels of IFN-γ (IFN gamma Mouse ELISA Kit, catalog no. BMS606; Thermo Fisher Scientific) and IL-17 (IL-17 Mouse ELISA Kit, catalog no. BMS6001; Thermo Fisher Scientific) in the conjunctiva or CLNs were quantified in accordance with the manufacturer's protocols for the ELISA kits. The optical absorbance was measured at 450 nm with a microplate reader (Bio-Tek ELx800; Bio-Tek Instruments, Inc., Winooski, VT, USA), and the protein concentrations were calculated by using a standard curve.
Flow Cytometry Assays
Single cells isolated from the lymph nodes were stained for 30 minutes with FITC-conjugated anti-CD4 (1:200, 2.5 µg/mL; BD Biosciences) and PE/Cyanine7 anti-mouse CD69 Antibody (1:200, 1 µg/mL, catalog no. 104512; BioLegend, San Diego, CA, USA) and washed. They were then were resuspended with Cell Staining Buffer (catalog no. 420201; BioLegend). For intracellular cytokine staining, cells were restimulated with Cell Stimulation Cocktail (catalog no. 423303; BioLegend) for 5 hours. Cells were fixed with 4% paraformaldehyde and permeabilized with Intracellular Staining Permeabilization Wash Buffer (catalog no. 421002; BioLegend); they were then stained with PE anti-mouse IL-17A Antibody (1:100, 2 µg/mL, catalog no. 506903; BioLegend) and APC anti-mouse IFN-γ Antibody (1:100, 2 µg/mL, catalog no. 505810; BioLegend). Stained cells were analyzed with a CytoFLEX S Flow Cytometer (Beckman Coulter, Indianapolis, IN, USA). Flow cytometry data were plotted and quantified with FlowJo software (FlowJo LLC, Ashland, OR, USA).
RNA Extraction and Real-Time PCR
Total RNA of the conjunctiva or CLNs was extracted using a PicoPure RNA Isolation Kit (catalog no. KIT0204; Thermo Fisher Scientific) and stored at –80°C. One sample consisted of the pooled conjunctiva of both eyes or CLNs of one animal. The RNA (1 µg) was reverse transcribed into cDNA using a reverse transcription kit (catalog no. RR047A; Takara Bio, Shiga, Japan). Real-time PCR was conducted with a SYBR Premix ExTaq Kit (catalog no. RR420A; Takara Bio). The primer sequences of the genes are listed in the Table. Each reaction (total volume, 10 µL) consisted of 1 µL of cDNA, 0.2 µL of each forward and reverse primer, 5 µL of reaction mixture (Takara Bio), and 3.6 µL of nuclease-free water. A three-step amplification program was utilized for the real-time PCR as follows: 45 cycles of 95°C for 10 seconds, 60°C for 5 seconds, and 72°C for 20 seconds. The PCR products were analyzed with a melting curve analysis performed after each LightCycler run.
Table.
Mouse Primer Sequences Used for Quantitative RT-PCR
| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| β-actin | CCTAAGGCCAACCGTGAAAAG | AGGCATACAGGGACAGCACAG |
| IFN-γ | AAATCCTGCAGAGCCAGATTAT | GCTGTTGCTGAAGAAGGTAGTA |
| IL-17 | CGCAATGAAGACCCTGATAGAT | CTCTTGCTGGATGAGAACAGAA |
| RORγt | AAGGCAAATACGGTGGTGTGG | CAGGACGGTTGGCATTGATGA |
| T-bet | CTAAGCAAGGACGGCGAATGT | CTTTCCACACTGCACCCACTT |
Statistical Analysis
The Mann–Whitney t-test was applied to analyze the data. P < 0.05 was considered statistically significant. Calculations were performed using Prism 7 software (GraphPad Software, Inc., San Diego, CA, USA). The probability of rejecting an incorrect hypothesis is known in statistics as the test of power (1 – β). Generally, the test of power is required to be above 0.8. The power calculation of every statistical analysis in this study was greater than 0.8. Because a type II error typically does not happen, we determined that five mice would be a proper sample size.
Results
The CD4+ T Cell-Mediated Inflammatory Response Was Activated by BAC in the Ocular Surface
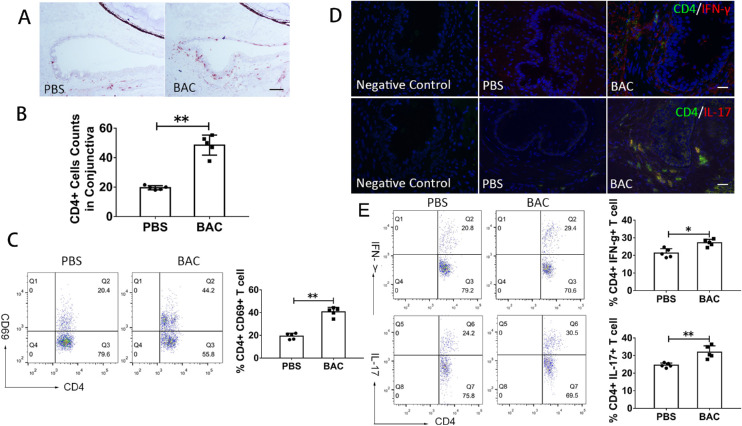
The effects of topical administration of 0.075% BAC on CD4+ T cells on the ocular surface of mice were examined. After 7 consecutive days, more CD4+ T cells infiltrated the conjunctiva in the BAC treatment group (48.53 ± 10.04) than in the PBS group (19.42 ± 2.23) (P = 0.008) (Figs. 1A, 1B). In CD4+ T cells isolated from draining nodes, flow cytometry (FCM) analysis showed that CD69+ T cells were significantly upregulated after BAC treatment (from 19.22% ± 2.58% to 40.66% ± 3.90%) (P = 0.008) (Fig. 1C). These results indicate that CD4+ T cells were activated and upregulated after topical application of BAC. Immunofluorescence double staining further showed that the ocular mucosal tissue was infiltrated by many Th1 and Th17 cells, which were absent in the PBS group (Fig. 1D). FCM analysis also showed that, in CD4+ T cells, IFN-γ+ cells and IL-17+ cells slightly increased after BAC treatment (from 21.3% ± 2.52% to 27.16% ± 1.96%, P = 0.016; from 24.5% ± 1.25% to 31.9% ± 3.61%, P = 0.008, respectively) (Fig. 1E).
Figure 1.
Effects of BAC on the infiltration of CD4+ T cells in the conjunctiva and CLNs. (A) Representative image of CD4+ T cell staining in the conjunctiva. Scale bars: 50 µm. (B) Statistical analysis of the number of CD4+ T cells in the conjunctiva. The data are shown as the mean ± SD. **P < 0.01 (n = 5). (C) Representative FCM plots of CD4+CD69+ T cells. (D) Representative image of IFN-γ+CD4+ and IL-17+CD4+ T cells in the conjunctiva. Scale bars: 50 µm. (E) Representative FCM plots of IFN-γ+CD4+ and IL-17+CD4+ T cells.
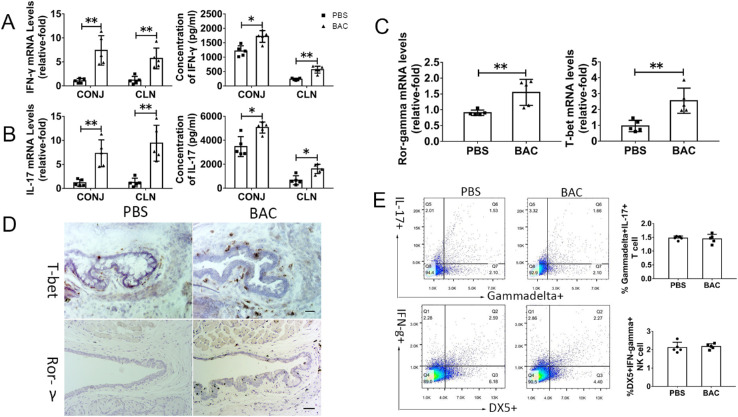
As evaluated by quantitative RT-PCR, mRNA expression of IFN-γ and IL-17 was dramatically upregulated in the conjunctival tissue (IFN-γ, 7.36 ± 2.75-fold, P = 0.008; IL-17, 8.11 ± 3.40-fold, P = 0.008) of BAC-treated mice compared with that of PBS-treated mice. IFN-γ and IL-17 protein levels in conjunctival tissue, as measured by ELISA, increased significantly to 1657.31 ± 295.23 pg/mL and 5060.58 ± 461.36 pg/mL in BAC-treated mice, respectively, levels that were 1.34-fold (P = 0.016) and 1.46-fold (P = 0.016) higher than those in PBS-treated mice (IFN-γ, 1236.70 ± 217.95 pg/mL; IL-17, 3472.36 ± 828.66 pg/mL) (Figs. 2A, 2B). Because CLNs are an important organ in the immune system,21,22 we also examined alterations of those cytokines in CLNs.
Figure 2.
Effects of BAC on CD4+ T cell-mediated inflammation in the conjunctiva and CLNs. (A) Statistical analysis of the gene expression and protein levels of IFN-γ in the conjunctiva (CONJ) and CLNs after topical application of BAC. (B) Statistical analysis of the gene expression and protein levels of IL-17 in the CONJ and CLNs after topical application of BAC. (C) Statistical analysis of gene expression of T-bet and RORγt in the conjunctiva. (D) Representative image of T-bet and RORγt staining in the conjunctiva. (E) Representative FCM plots of IL-17+gamma delta+ T cells and IFN-γ+DX5+NK/NKT cells. The data are shown as the mean ± SD. *P < 0.05, **P < 0.01 (n = 5).
Interestingly, IFN-γ and IL-17 were significantly increased at the mRNA level (IFN-γ, 6.39 ± 2.72-fold, P = 0.008; IL-17, 10.01 ± 4.04-fold, P = 0.008) and protein level (IFN-γ, 564.90 ± 120.19 pg/mL; IL-17, 1608.16 ± 387.46 pg/mL) in the BAC treatment group. These levels were 2.44-fold and 2.42-fold higher than those of the PBS-treated group (IFN-γ, 231.72 ± 35.04 pg/mL, P = 0.008; IL-17, 664.82 ± 375.40 pg/mL, P = 0.016) (Figs. 2A, 2B). These findings indicate that IFN-γ and IL-17 were largely stimulated in the ocular mucosa and draining CLNs in BAC-treated mice.
We then examined the expression of Th1-specific transcription factor T-bet and Th17-specific transcription factor retinoic acid-related orphan receptor gamma t (RORγt), which mediates Th1 and Th17 cell release of IFN-γ and IL-17. The gene expression of T-bet and RORγt was significantly upregulated by 1.76 ± 0.27-fold (P = 0.008) and 1.46 ± 0.21-fold (P = 0.008), respectively, in the conjunctiva of BAC-treated mice compared with those of PBS-treated mice (Fig. 2C). In the conjunctiva of BAC-treated mice, the expression of T-bet and RORγt dramatically increased, and they were weakly expressed in the PBS group, as determined by immunohistochemistry (Fig. 2D).
Natural killer (NK)/natural killer T (NKT) cells could be producing IFN-γ,23 and gamma delta T cells could be secreting IL-17.24 To determine whether IFN-γ and IL-17 were produced by NK/NKT cells and gamma delta T cells, we tested NK/NKT cells (DX5+) and gamma delta T cells (gamma delta TRC+) by FCM. There was no increase in NK/NKT cells or gamma delta T cells after BAC treatment (Fig. 2E). These results indicate that IFN-γ and IL-17 are secreted primarily by CD4+ T cells.
Taken together, these results suggest that topical administration of BAC results in CD4+ T cell infiltration and the release of Th1-secreted IFN-γ and Th17-secreted IL-17.
Ocular Surface Damage Was Observed Following Topical Application of BAC
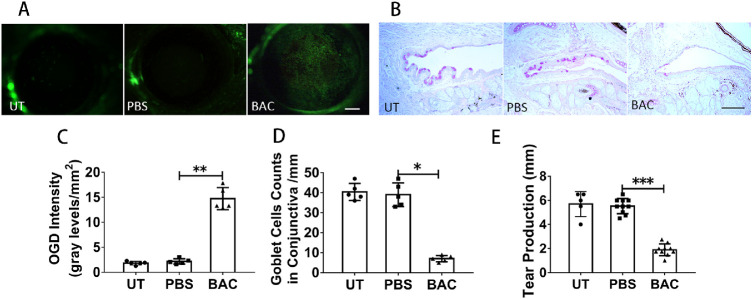
Because it has been reported that IL-17 induces corneal barrier dysfunction and IFN-γ results in the loss of conjunctival goblet cells,9,12 we then examined the ocular surface toxicity of BAC. We assessed corneal barrier dysfunction through OGD staining. There was widespread punctuate staining on BAC-treated mouse corneas (Fig. 3A), whereas there was little staining on PBS-treated and UT mouse corneas. The OGD intensity score showed that the average OGD intensity in the corneas of BAC-treated mice was 14.73 ± 2.28/mm2, which was dramatically higher than that in the PBS-treated group (2.17 ± 0.53/mm2) (P = 0.008) (Fig. 3C). PAS staining showed that the number of conjunctival goblet cells was significantly decreased from 39.0 ± 6.0 cells/mm for the PBS-treated group to 7.0 ± 1.6 cells/mm for the BAC-treated group (P = 0.04) (Figs. 3B, 3D). Tear production was decreased from 5.525 ± 0.63 mm for the PBS-treated mice (P = 0.001) to 1.9 ± 0.49 mm for the BAC-treated mice (Fig. 3E). These results indicate that topical application of 0.075% BAC induced ocular surface damage, including corneal barrier dysfunction, goblet cell loss, and reduced tear production.
Figure 3.
Effects of topical application of BAC on the ocular surface. (A) Representative images of OGD staining in the conjunctiva. Scale bars: 500 µm. (B) Representative images of PAS staining in the conjunctiva. Scale bars: 100 µm. (C) Statistical analysis of the OGD intensity data. (D) Statistical analysis of the mean number of goblet cells. (E) Statistical analysis of tear production. The data are shown as the mean ± SD. *P <0.05, **P <0.01, ***P < 0.001 (n = 5 or 10).
Similar Ocular Surface Damage and Inflammatory Cell Infiltration Were Induced by Adoptive Transfer of CD4+ T Cells in Nude Mice
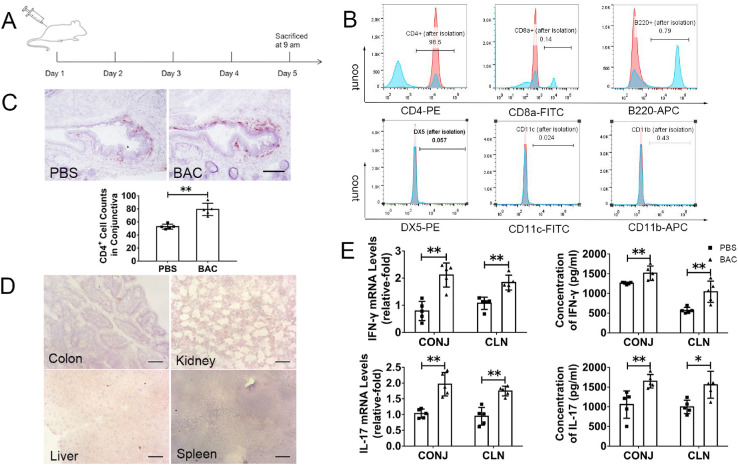
To evaluate the role of CD4+ T cells in the development of dry-eye-like ocular surface disorder, we conducted a classic adoptive transfer experiment in nude mice. CD4+ T cells isolated from C57BL/6 mice were subjected to BAC treatment and then adoptively transferred into nude mice. The effects of the transfer CD4+ T cells on the ocular surface in nude mice were then investigated on day 5 after transfer (Fig. 4A). FCM determined that the purity of the isolated CD4+ T cells was almost 95%. Non-CD4+ T cells (i.e., CD8+ T cells, B220+ B cells, DX5+ NK cells, CD11c+ dendritic cells, and CD11b+ macrophages) were almost completely depleted (each, <1%) (Fig. 4B). The control group consisted of nude mice that received CD4+ T cells isolated from PBS-treated C57BL/6 mice and UT nude mice. These three groups were referred to as BAC-treated nude mice, PBS-treated nude mice, and UT nude mice.
Figure 4.
Adoptive transfer of CD4+ T cells isolated from BAC-treated mice induced the infiltration of CD4+ T cells and activation of CD4+ T cell-mediated inflammation in nude mice. (A) Experimental design for adoptive transfer of CD4+ T cells. (B) The purity of the isolated CD4+ T cells was almost 95%, whereas the non-CD4+ T cells were almost depleted. The blue histograms show FCM analysis before isolation; the red histograms are after CD4+ isolation. The percentage of isolated cells in the CD4+ T cell fraction is depicted in the graph. (C) Representative image of CD4+ T cell staining in the conjunctiva and statistical analysis of the number of CD4+ T cells in the conjunctiva. Scale bars: 50 µm. (D) Representative image of CD4+ T cell staining in the colon, kidney, liver, and spleen. Scale bars: 50 µm. (E) Statistical analysis of gene expression and protein levels of IFN-γ and IL-17 in the conjunctiva and CLNs of nude mice. The data are shown as the mean ± SD. *P < 0.05, **P < 0.01 (n = 5).
CD4+ T cell infiltration in the conjunctiva in BAC-treated nude mice (78.85 ± 2.67 cells) was higher than that in PBS-treated nude mice (52.55 ± 2.12 cells) (P = 0.008) (Fig. 4C). The isolated CD4+ T cells did not migrate to the liver, kidney, spleen, or colon (Fig. 4D), verifying that CD4+ T cells had been successfully transferred into the nude mice. Furthermore, the gene expression levels of IFN-γ and IL-17 were significantly upregulated in BAC-treated nude mice compared with PBS-treated nude mice: in the conjunctiva, 2.05 ± 0.24-fold for IFN-γ (P = 0.008) and 1.99 ± 0.21-fold for IL-17 (P = 0.008); in CLNs, 1.83 ± 0.16-fold for IFN-γ (P = 0.008) and 1.75 ± 0.12-fold for IL-17 (P = 0.008). Similarly, the protein levels of IFN-γ and IL-17 were dramatically increased in BAC-treated nude mice compared with PBS-treated nude mice in the conjunctiva (for IFN-γ, from 1275.42 ± 50.99 pg/mL to 1657.31 ± 295.23 pg/mL, P = 0.008; for IL-17, from 1056.06 ± 346.84 pg/mL to 1649.00 ± 171.09 pg/mL, P = 0.008) and in CLNs (for IFN-γ, from 584.70 ± 75.82 pg/mL to 1007.73 ± 297.89 pg/mL, P = 0.008; for IL-17, from 1002.55 ± 195.01 pg/mL to 1551.11 ± 393.62 pg/mL, P = 0.016) (Fig. 4E). Adoptive transfer of CD4+ T cells that were isolated from BAC-treated mice induced the production of IFN-γ and IL-17 on the conjunctiva and in CLNs, similar to the direct effect of topical direct application of BAC. These results indicate the important role of CD4+ T cells in BAC toxicity.
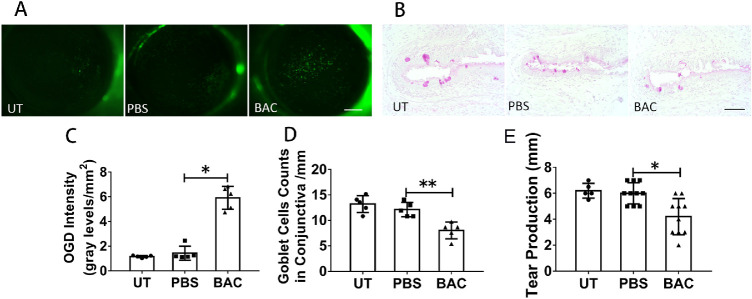
The ocular surface of the nude mice was then observed after adoptive transfer of CD4+ T cells. OGD staining showed that the mean intensity was increased to 5.91 ± 0.93/mm2 in BAC-treated nude mice compared to 1.423 ± 0.57/mm2 in the PBS-treated nude mice (P = 0.049) (Figs. 5A, 5C). PAS staining showed that the number of conjunctival goblet cells decreased from 12.2 ± 0.4 cells/mm for the PBS-treated nude mice (P = 0.004) to 8.013 ± 1.642 cells/mm for the BAC-treated nude mice (Figs. 5B, 5D). Tear production was decreased from 6.0 ± 0.82 mm for the PBS-treated nude mice (P = 0.017) to 4.2 ± 1.40 mm for the BAC-treated nude mice (Fig. 5E). These findings further indicate that only adoptive transfer CD4+ T cells isolated from the BAC-treated mice resulted in corneal barrier dysfunction, conjunctival goblet cell loss, and decreased tear production in the nude mice.
Figure 5.
Adoptive transfer of CD4+ T cells isolated from BAC-treated mice induced ocular surface damage in nude mice. (A) Representative images of OGD staining in the conjunctiva. Scale bars: 500 µm. (B) Representative images of PAS staining in the conjunctiva. Scale bars: 100 µm. (C) Statistical analysis of the OGD intensity data. (D) Statistical analysis of the mean number of goblet cells in the conjunctiva. (E) Statistical analysis of tear production. The data are shown as the mean ± SD. *P < 0.05, **P < 0.01 (n = 5 or 10).
Overall, the adoptive transfer of CD4+ T cells activated by BAC treatment led to similar inflammatory factor release and ocular surface disorder as the direct application of BAC.
Discussion
Preservatives are widely used in health care, household, cosmetic, and ophthalmic products.1 BAC is one of the most commonly used preservatives in ophthalmic solutions.2 The toxicity of BAC has been validated in clinical and experimental research,4,25,26 and it has been found that long-term use of eye drops with BAC causes redness, dryness, and foreign body sensation. BAC also induces tear film instability, cornea barrier dysfunction, and the loss of conjunctival goblet cells,27 which are similar to the clinical manifestation of dry eye syndrome. CD4+ T cells are key cells in the pathogenesis of dry eye syndrome; however, the underlying mechanisms by which BAC causes ocular surface damage are not completely understood.4,28 Herein, we have provided novel evidence that CD4+ T cells also play an important role in BAC-induced dry-eye-like damage to the ocular surface in C57BL/6 mice.
Adaptive immunity protects the body from attack by foreign objects. CD4+ T cells are crucial in adaptive immune regulation.29 When adaptive immunity occurs, CD4+ T cells are activated to release inflammatory factors against foreign objects. In the ocular surface, the conjunctiva acts as a barrier against the outer environment and selectively takes up antigens for immune protection.6 However, increased expression of inflammatory factors can not only kill antigens but also destroy normal cells. Th1 and Th17 cells are subsets of CD4+ T cells.30 Activated Th1 and Th17 cells can release IFN-γ and IL-17, which are highly upregulated in response to antigens and infections. IFN-γ and IL-17 are also expressed in autoimmune diseases, such as Sjögren's syndrome.31 Galletti's group17 reported that BAC broke down conjunctival immunological tolerance. We observed that CD4+ T cell infiltration and relative inflammatory factor release were increased in the conjunctiva and CLNs after BAC treatment, and our results indicate that BAC induces CD4+ T cell infiltration and expression of related cytokines on the ocular surface of mice.
According to DEWS II,6 dry eye disease is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles. Although dry eye disease is initiated by desiccating stress, the subsequent chain of inflammation events invites additional contributors to dry eye. We think that BAC-induced dry eye is an active process because (1) BAC reduced tear film stability,15 one of the core mechanisms of dry eye6; (2) BAC induced conjunctival epithelial apoptosis32 and inflammation33; and (3) BAC disrupted conjunctival immunological tolerance and activated the NF-κB signaling pathway after exposure to 0.01% BAC. We also observed maturation of dendritic cells and increased CD4+ T cells with BAC treatment.17 Therefore, we suggest that BAC induces epithelial cell death, then initiates a chain of inflammation events that results in activated CD4+ T cells aggravating ocular surface damage and causing dry eye.
Adoptive transfer experiments are usually performed to determine the role of T cells.6,34 Nude mice are deficient in T cells and are used to identify the immune response in animal dry eye models.6 Previous studies have found that when CD4+ T cells isolated from C57BL/6 mice exposed to desiccating stress were adoptively transferred to T cell-deficient nude mice, recipients had reduced tear production, corneal staining, and goblet cell loss.6,35 In this study, we isolated CD4+ T cells from C57BL/6 mice after treatment with BAC and adoptively transferred these cells into nude mice. Interestingly, we observed infiltration of CD4+ T cells into the conjunctiva and increased levels of CD4+ T cell-mediated immune inflammatory factors in the conjunctiva and CLNs in nude mice. In addition, ocular surface dry-eye-like damage was observed in the nude mice after transfer. These disorders in nude mice were consistent with the changes caused by direct topical application of BAC inC57BL/6 mice. This finding indicates that CD4+ T cells are crucial in BAC-induced ocular surface dry eye syndromes.
Glaucoma patients are often given BAC-containing formulations daily for years, leading to treatment-induced chronic inflammation and dry eye symptoms.25,35 There are many clinical side effects of long-term topical BAC treatment on the ocular surface, including morphological and phenotypical changes in epithelial cells,35,36 which have been verified by in vitro studies.32 Georgiev et al.15 observed an interaction between BAC and human tears, which could explain tear film instability after exposure to clinical concentrations of BAC. Liang et al.37 described increased inflammatory cell infiltration in the conjunctiva of rabbits treated with BAC. Such side effects of long-term BAC treatment affect the balance of the ocular surface microenvironment and result in dry eye. In our study, we found another mechanism, in that BAC can increase CD4+ T cells and further induce dry eye.
Previous studies have reported using BAC to induce dry eye in animal models at concentrations ranging from 0.1% to 0.4%,19,20,38 and corneal neovascularization appeared in both the 0.2% and 0.4% BAC treatment groups. In our study, we treated animals with BAC at concentrations of 0.05%, 0.075%, 0.1%, and 0.2%. We found that corneal neovascularization and corneal ulcers occurred in both the 0.1% and 0.2% BAC treatment groups (data not shown) but these conditions do not commonly occur in dry eye; therefore, we decided to use 0.075% BAC for our dry eye model.
In this study, we demonstrated that CD4+ T cells are induced and activated by topical application of BAC to the ocular surface; however, the detailed mechanisms of BAC-induced immunologic responses must be better understood. For example, when do immune responses occur after administration of BAC? What are the triggers that activate specific immunocytes? Do the effects disappear after BAC treatment? Further investigation is also required to elucidate the pathological changes.
Conclusions
This study provides new insights into the mechanism by which BAC damages the ocular surface. Topical application of BAC induces dry-eye-like ocular surface damage by activating the immune response. CD4+ T cells infiltrate the conjunctiva and release IFN-γ and IL-17 after BAC treatment, resulting in corneal barrier dysfunction, loss of conjunctival goblet cells, and reduced tear production.
Acknowledgments
Supported by Grants from the National Key R&D Program of China (2018YFA0107304, ZL) and the National Natural Science Foundation of China (81870627, ZL; 81900825, CH). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: W. Ouyang, None; Y. Wu, None; X. Lin, None; S. Wang, None; Y. Yang, None; L. Tang, None; Z. Liu, None; J. Wu, None; C. Huang, None; Y. Zhou, None; X. Zhang, None; J. Hu, None; Z. Liu, None
References
- 1. Baudouin C, Labbé A, Liang H, Pauly A, Brignolebaudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010; 29(4): 312–334. [DOI] [PubMed] [Google Scholar]
- 2. Shen J, Bejanian M. Effect of preservative removal from fixed-combination bimatoprost/timolol on intraocular pressure lowering: a potential timolol dose-response phenomenon. Clin Ophthalmol. 2016; 10: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li C, Song Y, Luan S, et al.. Research on the stability of a rabbit dry eye model induced by topical application of the preservative benzalkonium chloride. PLoS One. 2012; 7(3): e33688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeoun-Hee K, Jae-Chang J, Soon-Young J, Yu S, Won LK, Jeung PY. Comparison of the efficacy of fluorometholone with and without benzalkonium chloride in ocular surface disease. Cornea. 2016; 35(2): 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franklin RM, Remus LE. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Invest Ophthalmol Vis Sci. 1984; 25(2): 181–187. [PubMed] [Google Scholar]
- 6. Bron AJ, de Paiva CS, Chauhan SK, et al.. TFOS DEWS II pathophysiology report. Ocul Surf. 2017; 15(3): 438–510. [DOI] [PubMed] [Google Scholar]
- 7. Brandtzaeg P, Baekkevold ES, Farstad IN, et al.. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999; 20(3): 141–151. [DOI] [PubMed] [Google Scholar]
- 8. Stevenson W, Chauhan SK, Dana R.. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012; 130(1): 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Paiva CS, Villarreal AL, Corrales RM, et al.. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007; 48(6): 2553–2560. [DOI] [PubMed] [Google Scholar]
- 10. Xiaobo Z, Wei C, De Paiva CS, et al.. Interferon-γ exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011; 52(9): 6279–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014; 118: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paiva CSD, Chotikavanich S, Pangelinan SB, et al.. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009; 2(3): 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013; 32(1): 19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010; 3(5): 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgiev GA, Yokoi N, Koev K, et al.. Surface chemistry study of the interactions of benzalkonium chloride with films of meibum, corneal cells lipids, and whole tears. Invest Ophthalmol Vis Sci. 2011; 52(7): 4645–4654. [DOI] [PubMed] [Google Scholar]
- 16. Wilson WS, Duncan AJ, Jay JL. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol. 1975; 59(11): 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galletti JG, Gabelloni ML, Morande PE, et al.. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 2013; 6(1): 24–34. [DOI] [PubMed] [Google Scholar]
- 18. Lin Z, He H, Zhou T, et al.. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2013; 54(9): 6314–6325. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Chen W, Paiva CSD, et al.. Desiccating stress induces CD4 + T-cell-mediated Sjögren's syndrome-like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-γ. Invest Am J Pathol. 2011; 179(4): 1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao X, Luo P, Zhao H, et al.. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp Eye Res. 2013; 115: 31–40. [DOI] [PubMed] [Google Scholar]
- 21. Schaumburg CS, Siemasko KF, De Paiva CS, et al.. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011; 187(7): 3653–3662. [DOI] [PubMed] [Google Scholar]
- 22. Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013; 8(11): e78508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piersma SJ, Pak-Wittel MA, Lin A, Plougastel-Douglas B, Yokoyama WM. Activation receptor-dependent IFN-γ production by NK cells is controlled by transcription, translation, and the proteasome. J Immunol. 2019; 203(7): 1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coffelt SB, Kersten K, Doornebal CW, et al.. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015; 522(7556): 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossi GC, Tinelli C, Pasinetti GM, Milano G, Bianchi PE. Dry eye syndrome-related quality of life in glaucoma patients. Eur J Ophthalmol. 2009; 19(4): 572–579. [DOI] [PubMed] [Google Scholar]
- 26. Pisella PJ, Debbasch C, Hamard P, et al.. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004; 45(5): 1360–1368. [DOI] [PubMed] [Google Scholar]
- 27. Lin Z, Liu X, Zhou T, et al.. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011; 17: 257–264. [PMC free article] [PubMed] [Google Scholar]
- 28. Soriano-Romaní L, García-Posadas L, López-García A, Paraoan L, Diebold Y. Thrombospondin-1 induces differential response in human corneal and conjunctival epithelial cells lines under in vitro inflammatory and apoptotic conditions. Exp Eye Res. 2015; 134: 1–14. [DOI] [PubMed] [Google Scholar]
- 29. Walton S, Mandaric S, Oxenius A. CD4 T cell responses in latent and chronic viral infections. Front Immunol. 2013; 4: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma D, Zhu X, Zhao P, et al.. Profile of Th17 cytokines (IL-17, TGF-β, IL-6) and Th1 cytokine (IFN-γ) in patients with immune thrombocytopenic purpura. Ann Hematol. 2008; 87(11): 899–904. [DOI] [PubMed] [Google Scholar]
- 31. Voigt A, Bohn K, Sukumaran S, Stewart CM, Bhattacharya I, Nguyen CQ. Unique glandular ex-vivo Th1 and Th17 receptor motifs in Sjögren's syndrome patients using single-cell analysis. Clin Immunol. 2018; 192: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Debbasch C, Brignole F, Pisella PJ, Warnet JM, Rat P, Baudouin C.. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001; 42(3): 642–652. [PubMed] [Google Scholar]
- 33. Warcoin E, Clouzeau C, Roubeix C, et al.. Hyperosmolarity and benzalkonium chloride differently stimulate inflammatory markers in conjunctiva-derived epithelial cells in vitro. Ophthalmic Res. 2017; 58(1): 40–48. [DOI] [PubMed] [Google Scholar]
- 34. Niederkorn JY, Stern ME, Pflugfelder SC, et al.. Desiccating stress induces T cell-mediated Sjögren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176(7): 3950–3957. [DOI] [PubMed] [Google Scholar]
- 35. Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004; 111(12): 2186–2192. [DOI] [PubMed] [Google Scholar]
- 36. De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999; 40(3): 619–630. [PubMed] [Google Scholar]
- 37. Liang H, Baudouin C, Labbe A, Riancho L, Brignole-Baudouin F. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS One. 2012; 7(3): e33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Q, Zhang Y, Liu X, Wang N, Song Z, Wu K. A comparison of the effects of benzalkonium chloride on ocular surfaces between C57BL/6 and BALB/c mice. Int J Mol Sci. 2017; 18(3): 509. [DOI] [PMC free article] [PubMed] [Google Scholar]