Abstract
Renal cell carcinoma (RCC) is the most common malignancy in the kidney in the world, and the 5-year overall survival for patients remains poor due to the lack of effective treatment strategies. Although ABT-737, as a Bcl-2/Bcl-xL inhibitor, has recently emerged as a novel cancer therapeutic reagent, apoptosis induced by ABT-737 is often blocked in several types of cancer cells. This study investigated whether the combination of the small-molecule BH3 mimetic ABT-737 and the lysosome inhibitor chloroquine was an effective strategy for treating renal cancer cells. We found that the combination of ABT-737 and chloroquine synergistically decreased cell viability when compared to treatment with either single reagent. Cell apoptosis induced by a combined treatment was markedly inhibited by the caspase inhibitors z-DEVD-FMK and z-VAD-FMK. It was also inhibited by cathepsin inhibitor E-64 and CTSI (cathepsin inhibitor), which suggested that apoptosis was dependent on the cascade of caspase activation and cathepsins released from lysosomes. Furthermore, we found that ABT-737 could increase the cell level of ROS, which triggers cathepsin-mediated cell death and augments the role of chloroquine in cell death. So the combination of ABT-737 and chloroquine was an effective strategy for the treatment of renal cancer cells, and this combined strategy may widen the therapeutic window of ABT-737 and chloroquine as well as enhance the clinical efficacy of synergistic drug combinations.
Key words: ABT-737, Chloroquine, Renal cancer, Apoptosis, Combination treatment
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy in the kidney, representing 2–3% of human cancers (1). Despite the development of therapeutic modalities, the 5-year overall survival for patients of renal cancer remains poor (2). Antitumor drugs are generally recognized as inducers of cell death. Although new antitumor drugs are continually being developed, the lack of efficacy at systemically tolerable doses frequently eliminates their success in the clinic. In order to improve cellular response to a single antitumor drug, combination therapies are currently being utilized to lead to increased cancer cell death and increased free survival of patients (3).
One of the reasons for antitumor drug resistance is a low sensitivity of the tumor cells to apoptosis (4). With a self-amplifying mechanism, apoptosis can be induced through two pathways, the extrinsic pathway and the intrinsic pathway, which involves mitochondrial outer membrane permeabilization (MOMP), followed by cytochrome C release and the cascade of caspase activation (5,6). Despite the important role of mitochondria in cell apoptosis, more and more evidence suggests that another organelle, lysosomes, plays an important role as a point of proapoptotic signaling integration (7–9). Lysosomal membrane permeabilization (LMP) is organized as an early and initiating event in apoptosis triggered by apoptosis inducers; then cathepsins release cytoplasm from lysosomes and activate the cascade of caspases (10). So we want to know whether there is any interesting correlation between mitochondria and lysosomes for cell apoptosis.
In addition, the Bcl-2 family of proteins act as key regulators in the mitochondrial apoptosis pathway (11). Furthermore, certain Bcl-2 proteins are found localized in lysosomes, and Bcl-xL and Bax translocation to lysosomes had recently been reported, which affects LMP and cell apoptosis (12,13). ABT-737, as a small-molecule BH3 mimetic with very high affinity to Bcl-2, Bcl-xL, and Bcl-w, results in apoptosis of cancer cells. Nevertheless, ABT-737 was not cytotoxic, on its own, to many cancer cell lines (14). Chloroquine, an antimalarial drug, can accumulate in the lysosomes and increase the lysosomal volumes substantially, followed by destabilization of lysosomal membranes and the release of cathepsins from the lysosomal lumen, which induces caspase activation (15). In recent years, combination therapy for cancer has received increasing attention. In this study, we assess the combination effect of ABT-737 and chloroquine on renal cancer cell death.
MATERIALS AND METHODS
Cell Culture
Renal cancer cell lines A498 and 786-O were obtained from ATCC (Rockville, MD, USA), and the cell lines were cultured in 1640 supplemented with 10% FBS (Gibco, Carlsbad, CA, USA) inside an incubator containing 5% CO2 at 37°C.
General Reagents and Antibodies
ABT-737, z-VAD-FMK, z-DEVD-FMK, and z-LEHD-FMK were obtained from BioVision. Trolox and CTSI (cathepsin inhibitor) were obtained from Santa Cruz. E-64, chloroquine, and N-acetylcysteine were obtained from Sigma-Aldrich.
The antibodies used in this study are as follows: caspase 9 (Cat. #9508; Cell Signaling Technology), cathepsin B (Cat. #ab58802; Abcam), Bcl-2 (Cat. #ab692; Abcam), and Bcl-xL (Cat. #ab77571; Abcam).
Determination of Cell Viability
In images detected by fluorescence microscope, apoptotic cells were analyzed with GC3AI (an sfGFP-based caspase 3-like protease activation indicator) indicator as described previously (16), and propidium iodide (PI)-stained cells were considered to be necrosis cells. The cell viability after treatment with reagents was detected by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide] colorimetric assay. Renal cancer cells (1 × 104) were seeded in a 96-well plate and incubated for 24 h and then treated with various reagents for different periods of time. After treatment, the relative cell number was determined by MTT assay.
Expression Vectors and Cell Transfection and Transduction
Human Bcl-2 and Bcl-xL cDNA ORF were amplified by PCR and subcloned into pCDH-puro-CMV, and these sequences were confirmed by DNA sequencing. The cells A498 and 786-O were transfected with either construct plasmid or control plasmid for 48 h. The stable cell clones were selected in puromycin until individual colonies containing the transfected construct were confirmed by Western blot analysis. The siRNAs (shCaspase 9#1: CTTTGTGTCCTACTCTACTTT, shCaspase 9#2: CAGCTTCCAGATTGACGACAA, shCathepsin#1: CTGGTCAACTATGTCAACAACTC, shCathepsin#2: TTCACGTAAGATACAAGTTTCCTC) were synthesized and subcloned into a lentiviral siRNA vector.
Western Blot
All agents were purchased from Santa Cruz Biotechnology. The lysis buffer (1% SDS, 10 mM Tris-HCl, pH 7.6, 1 mM aprotinin, 1 mM leupeptin, and 1 mM BMSF) was used to obtain total protein, whose concentration was measured by Bradford method. Protein (20 mg) was separated on a 10% SDS-PAGE gel and blotted onto a PVDF membrane. Fat-free milk (5%) blocks the membrane, which was then incubated with primary antibody for 1 h and secondary antibody for 1 h at room temperature (17). β-Actin (sc-1616) was used as an internal control. Li-Cor Odyssey image reader (Li-Cor, USA) was used for Western blot detection.
Intracellular ROS Measurement
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA, 30 µM; Invitrogen) was used to measure intracellular ROS, which was oxidized to fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. Cells washed with PBS two times after treatment with reagents were incubated with 20 µM of H2DCF at 37°C for 30 min. To remove excess probe, the cells were washed with PBS again, and the fluorescence intensity was excited at 495 nm and measured at 527-nm emission wavelengths. Median fluorescent units were used for the analysis.
RESULTS
ABT-737 and Chloroquine Synergistically Induce Cell Death in Renal Cancer Cells
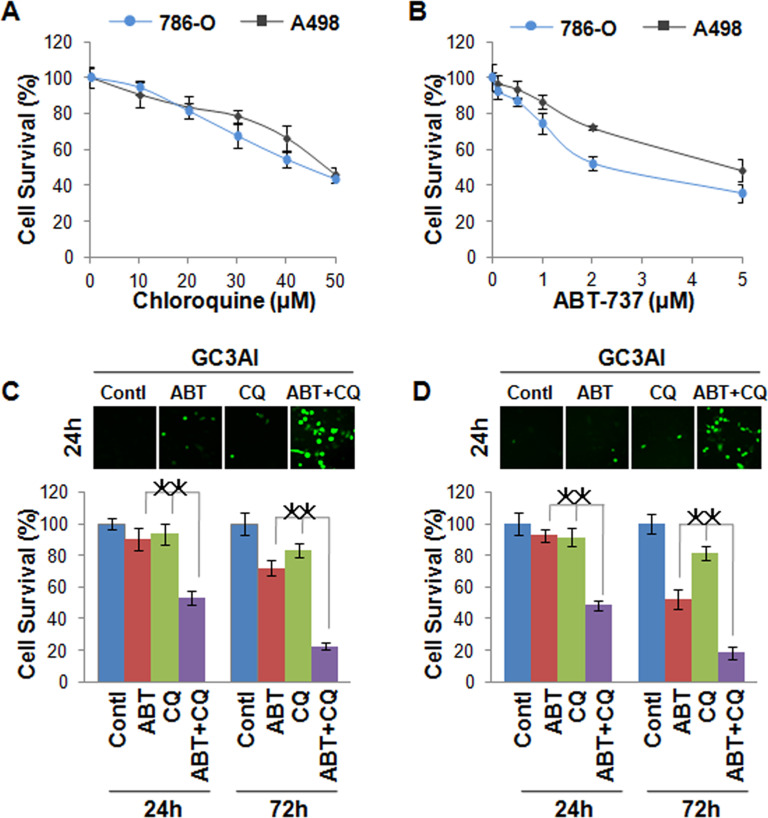
In this study, the renal cancer cells 786-O and A498 were used as cell models to detect the combination effect of ABT-737 and chloroquine on cell death. After treatment with ABT-737 for 48 h, cell death of both 786-O and A498 all increased in a dose-dependent manner detected by MTT (Fig. 1A). Similar results were found after treatment with chloroquine, and the cell death of both 786-O and A498 also increased (Fig. 1B). However, 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine showed very little activity as a single reagent. Combinatorial treatment is a very useful method in cancer chemotherapy. Here we investigated the synergistic effect of ABT-737 and chloroquine on cell death. Based on the above results, we selected the dose of 1 µmol/L of ABT-737 and the dose of 25 µmol/L of chloroquine in a 24-h treatment for the experiments. The apoptosis indicator GC3AI was utilized to detect cell apoptosis, which has fluorescent activity after cleavage by active caspase 3-like protease during apoptosis, and PI was used to monitor cell necrosis. Based on the images obtained by microscopy, the dose of 1 µmol/L of ABT-737 and the dose of 25 µmol/L of chloroquine alone induced only a few apoptotic cells in 24 h in 786-0 cells. But when we combined 1 µmol/L of ABT-737 with 25 µmol/L of chloroquine, more apoptotic cells were detected when compared with ABT-737 and chloroquine alone in 786-O in 24 h and more obviously in 72 h (Fig. 1C). In A498 cells, we found similar results, an obvious combined effect of ABT-737 and chloroquine on cell apoptosis (Fig. 1D).
Figure 1.
ABT-737 and chloroquine synergistically induce cell death in renal cancer cells. (A) The cell viability of 786-O and A498 cells after treatment with a different dose of chloroquine for 48 h detected by MTT; cell death increased in a dose-dependent manner. (B) The cell viability of 786-O and A498 cells after treatment with different doses of ABT-737 for 48 h detected by MTT; cell death increased in a dose-dependent manner. (C) Apoptosis of 786-O cells imaged by fluorescence microscope in 24 h (upper) and detected by MTT in 24 h and 72 h (lower) after treatment with 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or combination of these two reagents; 1 µmol/L of ABT-737 or 25 µmol/L of chloroquine alone induced only few apoptotic cells in 24 h and 72 h in 786-0 cells, but the combined treatment of ABT-737 and chloroquine induced more apoptotic cells. (D) Apoptosis of A498 cells imaged by fluorescence microscope in 24 h (upper) and detected by MTT in 24 h and 72 h (lower) after treatment with 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or a combination of these two reagents; 1 µmol/L of ABT-737 or 25 µmol/L of chloroquine alone induced only a few apoptotic cells, but the combined ABT-737 and chloroquine treatment induced more cell apoptosis. *p < 0.05.
ABT-737 and Chloroquine Synergistically Induce Lysosome-Dependent Cell Death in Renal Cancers
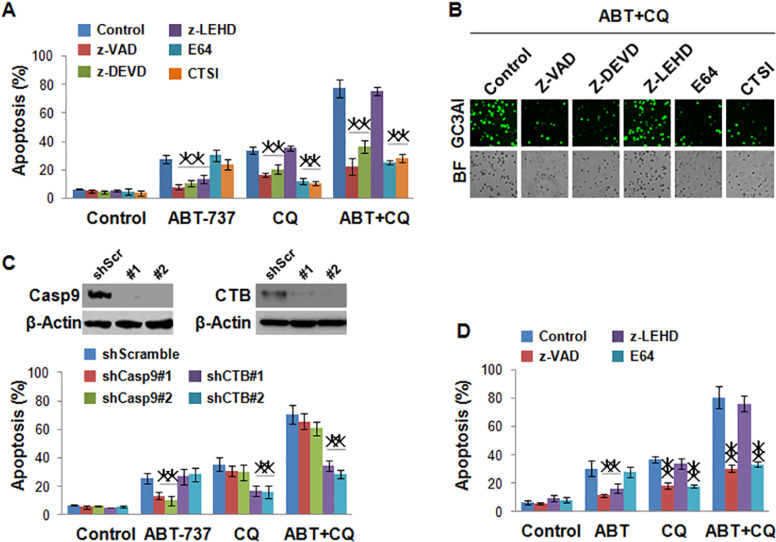
To investigate the mechanism of apoptosis induced by ABT-737 and chloroquine, pan-caspase inhibitor z-VAD, caspase 3/7 inhibitor z-DEVD, caspase 9 inhibitor z-LEHD, and lysosome protease inhibitors E-64 and CTSI were used. The results showed that cell death of A498 induced by chloroquine could be inhibited by all inhibitors, except for z-LEHD, suggesting that apoptosis induced by chloroquine was dependent on caspase 3/7 and lysosome protease, but not caspase 9. In addition, cell apoptosis induced by ABT-737 could be inhibited by all caspase inhibitors z-VAD, z-DEVD, and z-LEHD, but not by lysosome inhibitors E-64 and CTSI, which indicated that ABT-737 alone could not induce lysosome-dependent cell death in renal cell cancer. However, when combined with chloroquine, ABT-737 could obviously invigorate the inhibitory effect of chloroquine on cell viability, and cell apoptosis induced by the combination of ABT-737 and chloroquine was dependent on the lysosome (Fig. 2A). The images obtained by microscopy indicated the same results (Fig. 2B). In another renal cancer cell line, 786-0, we also found that ABT-737 and chloroquine synergistically induce lysosome-dependent cell death (Fig. 2D). To further confirm our hypothesis, we evaluated the effects of a knockdown of caspase 9 and cathepsin B on apoptosis induced by ABT-737 and chloroquine in A498. Shcaspase 9 decreased apoptosis that was induced by ABT-737, but not by chloroquine. However shCTB decreased apoptosis that was induced by chloroquine, but not by ABT-737 (Fig. 2C). All these results suggested that the combination treatment of ABT-737 with chloroquine leads to a dramatic apoptosis by increasing the activity of caspases and the release of cathepsins from the lysosomes.
Figure 2.
ABT-737 and chloroquine synergistically induce lysosome-dependent cell death in renal cancers. (A) The effect of caspase inhibitors and lysosome protease inhibitors on apoptosis of A498 cells induced by 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or combination of these two reagents in 72 h. The cell death induced by chloroquine could be inhibited by all inhibitors, except for z-LEHD; the cell death induced by ABT-737 could be inhibited by all caspase inhibitor but not by lysosome inhibitor. (B) Apoptotic cells of A498 imaged by fluorescence microscope induced by a combination of 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine with or without caspase inhibitors and lysosome protease inhibitors in 72 h. (C) The effect of knockdown of caspase 9 and cathepsin B (CTB) on the apoptosis of A498 induced by 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or combination of these two reagents in 72 h; shcaspase 9 decreased apoptosis induced by ABT-737; shCTB decreased apoptosis induced by chloroquine. (D) The effect of caspase inhibitors and lysosome protease inhibitors on apoptosis of 786-O cells induced by 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or a combination of these two reagents in 72 h. *p < 0.05, **p < 0.01.
Bcl-2 Proteins Protect Cell Death in Renal Cancers Induced by ABT-737 and Chloroquine
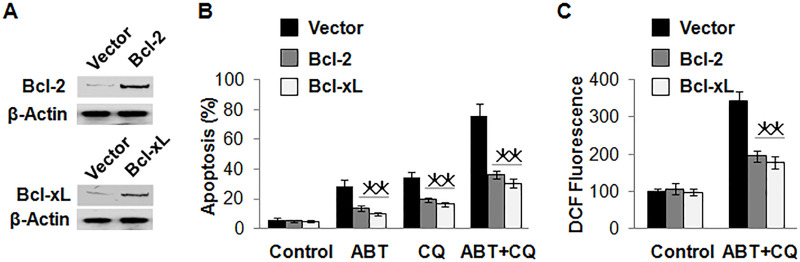
As we know, the Bcl-2 family of proteins acts as a key regulator in the mitochondrial apoptosis pathway. ABT-737 can induce cell apoptosis through banding to Bcl-2 and Bcl-xL directly with high affinity. Bcl-2-type antiapoptotic proteins, Bcl-2 and Bcl-xL, predominantly act by inhibiting apoptosis. Therefore, we speculated that overexpressed Bcl-2 and Bcl-xL restore cell apoptosis induced by ABT-737. In this study, Bcl-2 and Bcl-xL were overexpressed in renal cancer cells A498 and 786-O to view their effect on apoptosis induced by ABT-737 and chloroquine (Fig. 3A). As we expected, the number of apoptotic cells induced by ABT-737 was obviously decreased in A498 when Bcl-2 or Bcl-xL was overexpressed in these cells. Interestingly, overexpressed Bcl-2 or Bcl-xL also eliminated apoptosis induced by chloroquine alone or by a combination of ABT-737 and chloroquine (Fig. 3B).
Figure 3.
Bcl-2 proteins protect cell death in renal cancers induced by ABT-737 and chloroquine. (A) Overexpressed Bcl-2 or Bcl-xL in A498 cells detected using Western blot. (B) The effect of overexpressed Bcl-2 or Bcl-xL on apoptosis of A498 cells induced by 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or a combination of these two reagents in 72 h. The apoptosis was obviously decreased when Bcl-2 or Bcl-xL was overexpressed in these cells. (C) The effect of overexpressed Bcl-2 or Bcl-xL on ROS induction after treatment with a combination of ABT-737 and chloroquine. The production of ROS was obviously inhibited in cells overexpressing Bcl-1 or Bcl-xL. *p < 0.05.
ROS Plays a Critical Role in Cell Death in Renal Cancers Induced by ABT-737 and Chloroquine
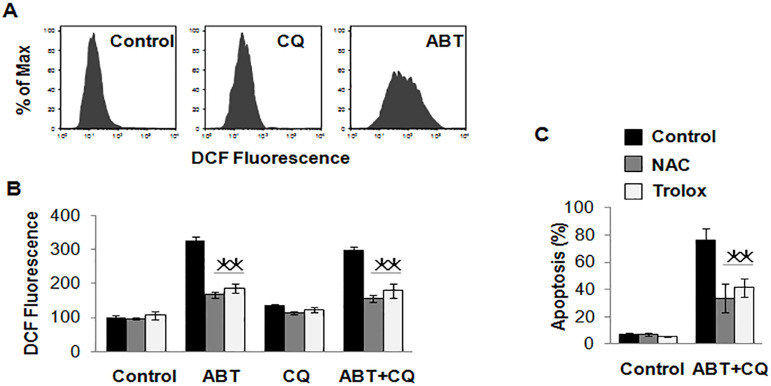
A recent study indicates that Bcl-2 could be an important ROS regulator (18). ROS could also disrupt the integrity of the lysosomes, which triggers caspase-mediated apoptosis or cathepsin-mediated necrosis (19). So we speculated that apoptosis induced by ABT-737 and chloroquine may relate to ROS in renal cancer cells. To confirm our speculation, we first detected the level of ROS in renal cancer cells overexpressing Bcl-1 or Bcl-xL using the method of DCF. The results showed that there was a higher level of ROS in control cells after treatment with ABT-737 and chloroquine, but the production of ROS was obviously inhibited in cells overexpressing Bcl-1 or Bcl-xL (Fig. 3C), which indicated a close correlation between ROS and apoptosis induced by a combination of ABT-737 and chloroquine. ABT-737 could induce the production of ROS, but chloroquine did not have this role (Fig. 4A). Then antioxidants N-acetylcysteine (NAC) and trolox were used to scavenge ROS produced by ABT-737 (Fig. 4B). NAC and trolox inhibited apoptosis induced by ABT-737 and chloroquine successfully (Fig. 4C).
Figure 4.
ROS plays a critical role in cell death in renal cancers induced by ABT-737 and chloroquine. (A) The effect of ABT-737 and chloroquine on ROS induction in A498 cells, and ABT-737 could induce the production of ROS. (B) The effect of NAC and trolox on ROS of A498 cells induced by 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine alone or combination of these two reagents in 72 h. NAC and trolox were used to scavenge ROS produced by ABT-737. (C) The effect of ROS inhibitors on apoptosis of A498 cells induced by a combination of 1 µmol/L of ABT-737 and 25 µmol/L of chloroquine. NAC and trolox inhibited the cell apoptosis. *p < 0.05.
DISCUSSION
In this study, we investigated the combined effect of ABT-737 and chloroquine on cell death of renal cancer cells. The results showed that ABT-737 and chloroquine synergistically induced cell death, which were dependent lysosomes. Further studies suggested that ABT-737 could induce the production of ROS, and ROS disrupts the integrity of the lysosomes. However, further studies are still needed to investigate its underlying mechanism.
Renal cell carcinoma is the most common malignancy of the kidney, representing 3% of human cancers, and approximately 25% of patients with renal cell carcinoma present distant metastases at diagnosis (1). Localized renal cell carcinoma is often curable by surgery alone, but metastatic renal cell carcinoma is generally incurable. Although combining conventional therapies, including radiation and chemotherapy, responses were seen in only a minority of highly select patients. In addition, targeted therapy has been introduced recently, but there is no obvious improvement, and most patients eventually relapse (20). Thus, managing advanced renal cell carcinoma remains one of the most significant challenges to clinicians, and searching for effective antitumor drugs is essential to improve the prognosis of renal cell carcinoma patients.
Normally, an attractive novel approach for antitumor therapeutics is to overcome resistance to apoptosis because impaired apoptosis renders the tumor cell more resistant to conventional cytotoxic therapy. Chemotherapeutic drugs could induce mitochondrial apoptosis, which determines the drug response. Mitochondrial apoptosis is mainly regulated by the Bcl-2 family of proteins. ABT-737, as a Bcl-2 homology 3 (BH3) mimetic, can induce cell apoptosis (21). Nevertheless, ABT-737 was not cytotoxic on its own in many cancer cell lines because of the different expression levels of the Bcl-2 family of proteins, especially Mcl-1 (22). In this study, less than 10% of renal cancer cells underwent apoptosis in the 24 h following treatment with only 1 µM/L of ABT-737. In view of this, combining ABT-737 with another available therapeutic drug may well provide substantial clinical benefit.
Chloroquine, as an old and safe drug, has long been used to treat malaria and autoimmune diseases in many countries (23). Many studies show that chloroquine accumulating within the lysosome leads to less acidic conditions and thereby decreased lysosomal function. More and more evidence indicates that chloroquine sensitizes cancer cells to radiation and other anticancer drugs, and this function has been well documented in experimental animal models (24–26). The synergistic anticancer effect of lidamycin and chloroquine on non-small cell lung cancer cells in vitro results has been reported, through a caspase-dependent pathway and inhibition of cytoprotective autophagy (27). But there was no synergistic anticancer effect of ABT-737 and chloroquine on non-small cell lung cancer because the combination of ABT-737 with chloroquine could lead to significant inhibition of Mcl-1 and marked upregulation of protein NOXA, both of which would serve to facilitate apoptosis (28). In contrast, the synergistic anticancer effects of ABT-737 and chloroquine on both 786-O and A498 renal cancer cells were obvious, and approximately six- to sevenfold cell apoptosis increases were seen after combination treatment, in contrast to after ABT-737 or chloroquine alone in a low dose in our study. The study indicated that the effect of a combination of ABT-737 and chloroquine on cell death may be different in different cell lines.
A critical but challenging task with any new therapeutic reagent is determining its biological mechanism of action. The precise mechanism by which chloroquine exerts anticancer effects is unclear for now. One reason is that inhibition of lysosome activity by chloroquine arrests the degradation of the autolysosome, which results in failure to provide energy through the autophagy pathway (29). In this study, cell apoptosis induced by high-dose ABT-737 could be inhibited by all caspase inhibitors z-VAD, z-DEVD, and z-LEHD, and overexpressed Bcl-2 and Bcl-xL could also protect apoptosis induced by ABT-737, which is consistent with other reports. Since ABT-737 can affect cell functions, and certain Bcl-2 proteins are found localized in lysosomes and affect LMP, which induce the cell apoptosis-dependent lysosomes, we suggest that ABT-737 could affect the apoptosis induced by cathepsins released from lysosomes. But cathepsin inhibition of both E64 and CTSI could not inhibit apoptosis induced by ABT-737, so we indicate that apoptosis induced by ABT-737 is independent of the protease released from lysosomes. Low-dose 25 µM chloroquine induced only few apoptotic cells in our study, so what is the material mechanism of the combination effect of ABT-737 and chloroquine in inducing cell death in renal cancer cells synergistically?
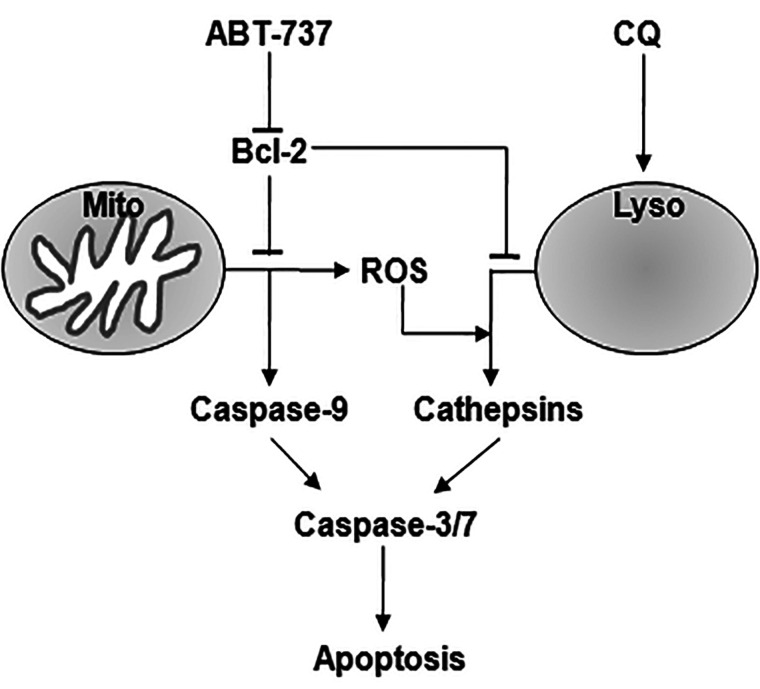
ROS has been shown to play a vital role in different cellular processes, such as proliferation, gene expression, and differentiation. More evidence indicates that ROS generation plays an important role in apoptosis of cancer cells. By interacting with the Bcl-2 family of proteins, ROS could activate mitochondrial intrinsic apoptotic cascade. In addition, ROS could disrupt the integrity of the lysosomes and then trigger cathepsin-mediated necrotic cell death. In our study, a higher level of ROS was detected after treatment with ABT-737 and chloroquine. ROS inhibitors, both NAC and trolox, inhibited apoptosis induced by ABT-737 and chloroquine successfully. So our results suggest that, on one hand, ABT-737 could induce cell apoptosis by inhibiting prosurvival Bcl-2 proteins and activating caspases directly; on the other hand, ABT-737 could increase the cell level of ROS produced by mitochondrial, which triggers cathepsin-mediated cell death and augments the role of chloroquine on cell death (Fig. 5).
Figure 5.
Working model for synergistic cell death induced by ABT-737 and chloroquine. On one hand, ABT-737 could induce cell apoptosis by inhibiting prosurvival Bcl-2 proteins and activating caspases directly; on the other hand, ABT-737 could increase the cell level of ROS produced by mitochondrial, which triggers cathepsin-mediated cell death and augments the role of chloroquine on cell death.
Several studies showed a favorable effect of chloroquine as a novel antitumor drug in other cancers because of its inhibitory action on autophagy (30). However, autophagy is also considered to have a cancer-suppressing role (31). A combination of chloroquine and other chemotherapeutic drugs could induce a toxic effect not only on cancer cells but also on kidney cells and other organs (32). So clinicians in the field of cancer therapy should pay more attention to the possibility that chloroquine has this same side effect on other organs.
REFERENCES
- 1. Jemal A.; Bray F.; Center M. M.; Ferlay J.; Ward E.; Forman D. Global cancer statistics. CA Cancer J. Clin. 61:69–90; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Gupta K.; Miller J. D.; Li J. Z.; Russell M. W.; Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat. Rev. 34:193–205; 2008. [DOI] [PubMed] [Google Scholar]
- 3. Skarzynski M.; Niemann C. U.; Lee Y. S.; Martyr S.; Maric I.; Salem D.; Stetler-Stevenson M.; Marti G. E.; Calvo K. R.; Yuan C.; Valdez J.; Soto S.; Farooqui M. Z.; Herman S. E.; Wiestner A. Interactions between ibrutinib and anti-CD20 antibodies: Competing effects on the outcome of combination therapy. Clin. Cancer Res. 22:86–95; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inoue S.; Salah-Eldin A. E.; Omoteyama K. Apoptosis and anticancer drug resistance. Human Cell 14:211–221; 2001. [PubMed] [Google Scholar]
- 5. Cosentino K.; Garcia-Saez A. J. Mitochondrial alterations in apoptosis. Chem. Phys. Lipids 181:62–75; 2014. [DOI] [PubMed] [Google Scholar]
- 6. Elkholi R.; Renault T. T.; Serasinghe M. N.; Chipuk J. E. Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab. 2:16; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boya P.; Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene 27:6434–6451; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Boya P.; Gonzalez-Polo R. A.; Poncet D.; Andreau K.; Vieira H. L.; Roumier T.; Perfettini J. L.; Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22:3927–3936; 2003. [DOI] [PubMed] [Google Scholar]
- 9. Blomgran R.; Zheng L.; Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukoc. Biol. 81:1213–1223; 2007. [DOI] [PubMed] [Google Scholar]
- 10. Mnich K.; Carleton L. A.; Kavanagh E. T.; Doyle K. M.; Samali A.; Gorman A. M. Nerve growth factor-mediated inhibition of apoptosis post-caspase activation is due to removal of active caspase-3 in a lysosome-dependent manner. Cell Death Dis. 5:e1202; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasi N.; Hwang M.; Jaboin J.; Csiki I.; Lu B. Regulated cell death pathways: New twists in modulation of BCL2 family function. Mol. Cancer Ther. 8:1421-1429; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kagedal K.; Johansson A. C.; Johansson U.; Heimlich G.; Roberg K.; Wang N. S.; Jurgensmeier J. M.; Ollinger K. Lysosomal membrane permeabilization during apoptosis—Involvement of Bax? Int. J. Exp. Pathol. 86:309–321; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oberle C.; Huai J.; Reinheckel T.; Tacke M.; Rassner M.; Ekert P. G.; Buellesbach J.; Borner C. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 17:1167–1178; 2010. [DOI] [PubMed] [Google Scholar]
- 14. Kline M. P.; Rajkumar S. V.; Timm M. M.; Kimlinger T. K.; Haug J. L.; Lust J. A.; Greipp P. R.; Kumar S. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia 21:1549–1560; 2007. [DOI] [PubMed] [Google Scholar]
- 15. Fan C.; Wang W.; Zhao B.; Zhang S.; Miao J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg. Med. Chem. 14:3218–3222; 2006. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J.; Wang X.; Cui W.; Wang W.; Zhang H.; Liu L.; Zhang Z.; Li Z.; Ying G.; Zhang N.; Li B. Visualization of caspase-3-like activity in cells using a genetically encoded fluorescent biosensor activated by protein cleavage. Nat. Commun. 4:2157; 2013. [DOI] [PubMed] [Google Scholar]
- 17. Cui Y.; Yang S.; Fu X.; Feng J.; Xu S.; Ying G. High levels of KAP1 expression are associated with aggressive clinical features in ovarian cancer. Int. J. Mol. Sci. 16:363–377; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Z. X.; Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 17:408–420; 2010. [DOI] [PubMed] [Google Scholar]
- 19. Kurz T.; Terman A.; Gustafsson B.; Brunk U. T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta 1780:1291–1303; 2008. [DOI] [PubMed] [Google Scholar]
- 20. Coppin C.; Kollmannsberger C.; Le L.; Porzsolt F.; Wilt T. J. Targeted therapy for advanced renal cell cancer (RCC): A Cochrane systematic review of published randomised trials. BJU Int. 108:1556–1563; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Zall H.; Weber A.; Besch R.; Zantl N.; Hacker G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol. Cancer 9:164; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woo S. M.; Min K. J.; Seo B. R.; Nam J. O.; Choi K. S.; Yoo Y. H.; Kwon T. K. Cafestol overcomes ABT-737 resistance in Mcl-1-overexpressed renal carcinoma Caki cells through downregulation of Mcl-1 expression and upregulation of Bim expression. Cell Death Dis. 5:e1514; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiesner J.; Ortmann R.; Jomaa H.; Schlitzer M. New antimalarial drugs. Angew. Chem. Int. Ed. Engl. 42:5274–5293; 2003. [DOI] [PubMed] [Google Scholar]
- 24. Njaria P. M.; Okombo J.; Njuguna N. M.; Chibale K. Chloroquine-containing compounds: A patent review (2010-2014). Expert Opin. Ther. Pat. 25:1003–1024; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang M. C.; Wu M. Y.; Hwang M. H.; Chang Y. T.; Huang H. J.; Lin A. M.; Yang J. C. Chloroquine enhances gefitinib cytotoxicity in gefitinib-resistant nonsmall cell lung cancer cells. PLoS One 10:e0119135; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimura T.; Shirakawa R.; Yaoita N.; Hayashi T.; Nagano K.; Horiuchi H. The antimalarial drugs chloroquine and primaquine inhibit pyridoxal kinase, an essential enzyme for vitamin B6 production. FEBS Lett. 588:3673–3676; 2014. [DOI] [PubMed] [Google Scholar]
- 27. Liu F.; Shang Y.; Chen S. Z. Chloroquine potentiates the anti-cancer effect of lidamycin on non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 35:645–652; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zinn R. L.; Gardner E. E.; Dobromilskaya I.; Murphy S.; Marchionni L.; Hann C. L.; Rudin C. M. Combination treatment with ABT-737 and chloroquine in preclinical models of small cell lung cancer. Mol. Cancer 12:16; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimura T.; Takabatake Y.; Takahashi A.; Isaka Y. Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 73:3–7; 2013. [DOI] [PubMed] [Google Scholar]
- 30. Yang Y.; Karakhanova S.; Hartwig W.; D’Haese J. G.; Philippov P. P.; Werner J.; Bazhin A. V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell. Physiol.; 2016. doi: 10.1002/jcp.25349; 2016 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 31. Takamura A.; Komatsu M.; Hara T.; Sakamoto A.; Kishi C.; Waguri S.; Eishi Y.; Hino O.; Tanaka K.; Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25:795–800; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi A.; Kimura T.; Takabatake Y.; Namba T.; Kaimori J.; Kitamura H.; Matsui I.; Niimura F.; Matsusaka T.; Fujita N.; Yoshimori T.; Isaka Y.; Rakugi H. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 180:517–525; 2012. [DOI] [PubMed] [Google Scholar]