Abstract
MicroRNAs (miRNAs) have been shown to be involved in bladder cancer progression. miR-489 (also known as miR-489-3p) was recently reported to be a tumor suppressor in several cancers. However, its exact role and mechanism in the progression of bladder cancer are largely unknown. In this study, we explore the role of miR-489 in the proliferation and invasion of human bladder cancer cells. The miR-489 expression levels were detected in bladder cancer and normal adjacent tissues, as well as in human normal bladder epithelial cells and bladder cancer cell lines. The results showed that miR-489 was sharply reduced in bladder cancer tissues and cell lines. Then the miR-489 mimic or oligo anta-miR-489 was transfected into T24 and UMUC3 bladder cancer cell lines. The results showed that the miR-489 mimic greatly increased the miR-489 level and significantly decreased the proliferation and invasion of T24 and UMUC3 cells. In contrast, the anta-miR-489 had a completely opposite effect on miR-489 expression, cell proliferation, and cell invasion. Moreover, bioinformatics and luciferase reporter gene assays confirmed that miR-489 targeted the mRNA 3′-untranslated region (3′-UTR) region of Jagged1 (JAG1), a Notch ligand. In conclusion, miR-489 suppressed proliferation and invasion of human bladder cancer cells.
Key words: miR-489, Bladder cancer, Proliferation, Invasion, JAG1
INTRODUCTION
Bladder cancer, the most common malignant tumor in the urinary system, is a frequent and highly mortal malignancy with an incidence of 429,800 new cases/year and 165,100 deaths (1,2). Because of the lack of sufficient attention and research funds, improvements in bladder cancer therapy are far less than expected (2). It is urgent that novel therapeutic targets and strategies are developed.
The Notch signaling pathway is a highly conserved signaling system present in higher animals (3,4). Notch signaling plays a major role in the regulation of embryogenesis, nervous system development and function, cardiovascular development, endocrine development, and tumorigenesis (4–6). The Notch cascade consists of Notch and Notch ligands as well as intracellular secondary transductors. Mammalian Notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4, are transmembrane proteins with extracellular and intracellular domains (4,7). They are bound by ligand proteins at the extracellular domain and then induce proteolytic cleavage and intracellular domain released to modify gene expression (8). Notch signaling is dysregulated in many cancers, and blockade of notch signaling has been shown to have antitumorigenic effects (3,9,10).
MicroRNAs (miRNAs or miRs) are small noncoding RNAs that negatively regulate expression-specific genes by targeting their 3′-untranslated regions (3′-UTRs) (11). Through targeting various genes, miRNAs play an important role in diverse biological processes, including normal tissue development and tumor progression (12–15). miR-489 (also known as miR-489-3p) was recently reported to be a tumor suppressor in several cancers. miR-489 was downregulated in non-small cell lung cancer tissues and cells, and downregulation of miR-489 increased cell invasion (16). In breast cancer, miR-489 expression was downregulated by about six- to sevenfold, and miR-489 overexpression in breast cancer resulted in increased cell death and cell cycle arrest (17). Moreover, miR-489 was one of the downregulated miRNAs in hypopharyngeal squamous cell carcinoma (HSCC) and had an inhibitory effect on HSCC cell proliferation (18). However, the exact role and mechanism of miR-489 in the progression of bladder cancer are largely unknown.
In this study, miR-489 expression was detected in bladder cancer and normal adjacent tissues, as well as in human normal bladder epithelial cells and bladder cancer cell lines. We found that miR-489 was sharply reduced in cancerous tissues and cell lines. Oligo miR-489 mimic and anta-miR-489 were, respectively, transfected into T24 and UMUC3 human bladder cancer cell lines. The effect of miR-489 on cell proliferation and invasion was investigated, and the underlying mechanism was explored.
MATERIALS AND METHODS
Ethical Statements
This study protocol was approved by the Ethics Committee of the Shaanxi Provincial People’s Hospital. The subjects were previously informed of the experimental details and gave written consent.
Tissue Sampling
Bladder cancer and matched normal adjacent tissues were isolated from a total of 48 bladder cancer patients (54 ± 6.8 years) who underwent tumor resection during 2013 to 2015 in Shaanxi Provincial People’s Hospital. Patients who had ever received chemo- or radiotherapy were excluded from this study.
Cell Culture and Transfection
Human normal bladder epithelial cell SV-HUC-1; human bladder cancer cell lines, including BIU-87, EJ, T24, 5637, and UMUC3; and HEK293 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin (Gibco), and 100 U/ml streptomycin (Gibco). The cells were incubated at a humidified atmosphere at 37°C in a 5% CO2 incubator.
For transfection, T24 and UMUC3 bladder cancer cells were subcultured in six-well plates. Upon reaching 80% of confluence, 80 nM oligo miR-489 mimic, 80 nM anta-miR-489 oligo, or 80 nM relative control oligos was transfected into the cells by using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The miR-489 mimic, anta-miR-489, and their control oligos were designed and synthesized by Ribobio Co. Ltd. (Guangzhou, China).
Cell Proliferation
The cells were seeded in six-well culture plates at a density of 1 × 105 cells/per well. After transfection for 48 h, cell proliferation was evaluated by using the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer’s instructions.
In Vitro Invasion Assay
Transwell invasion assay was used to detect cell invasion. Cells (5 × 104) with serum-free medium were plated in the top chamber with a noncoated membrane (24-well insert; 8 μm; Corning). Medium containing 10% serum was used as a chemoattractant in the lower chamber. After incubation for 24 h, a cotton swab was used to remove the nonmigrated cells in the upper chamber, and the filters were individually stained with 2% crystal violet. The invasive cells were captured under a light microbladder cancer scope (×100; Olympus IX70; Olympus Corporation, Osaka, Japan) and counted.
Real-Time Quantitative PCR
Real-time quantitative polymerase chain reactions (qPCRs) were carried out in a 25-μl system, using SYBR Premix Ex Taq (TaKaRa, Dalian, China), 0.4 mM primers, and 200 ng of cDNA template. The primers of miR-489 and U6 RNA (as internal reference) were designed and produced by Ribobio Co. Ltd. PCR amplification cycles were performed using the SYBR Premix Ex Taq II Kit (Invitrogen). The reactions were initially denatured at 95°C for 2 min, followed by 35 cycles of 95°C for 15 s and 55°C for 1 min. The transcript abundance was calculated using the 2−ΔΔCt method.
Western Blotting
Fifty micrograms of total protein from each sample was separated by 12% SDS-PAGE. The protein was transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) for immunoblotting analysis. The primary antibodies anti-Jagged1 (JAG1) (1:300; Abcam) and anti-GAPDH (1:500; Abcam) were used to incubate with the proteins on the PVDF membrane at 4°C overnight. After incubation with the horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature, proteins were detected with an ECL kit (Millipore) and analyzed with Quantity One analysis software (Bio-Rad).
3′-UTR Luciferase Gene Reporter Assay
The 3′-UTR region of the human JAG1 mRNA was amplified by PCR. The mutant JAG1 3′-UTR was amplified by PCR and created by a Site-Directed Mutagenesis Kit (TaKaRa). The wild-type (WT) and mutant sequences were, respectively, purified and inserted into a psiCHECK-2 vector (Promega, Madison, WI, USA). The control mimic and miR-489 mimic were, respectively, cotransfected with psiCHECK-JAG1 3′-UTR into the HEK293 cells by using the X-tremeGENE 9 DNA Transfection Reagent (Roche, Basel, Switzerland). The cells were then incubated for 72 h, and luciferase activity was measured using a microplate reader (PT-3502; Potenov, Beijing, China).
Statistical Analysis
All data were obtained from at least three independent experiments. The data were expressed as means ± SEM. Statistics were calculated with SPSS v19.0. Multiple comparisons were assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s tests. A value of p < 0.05 was considered statistically significant.
RESULTS
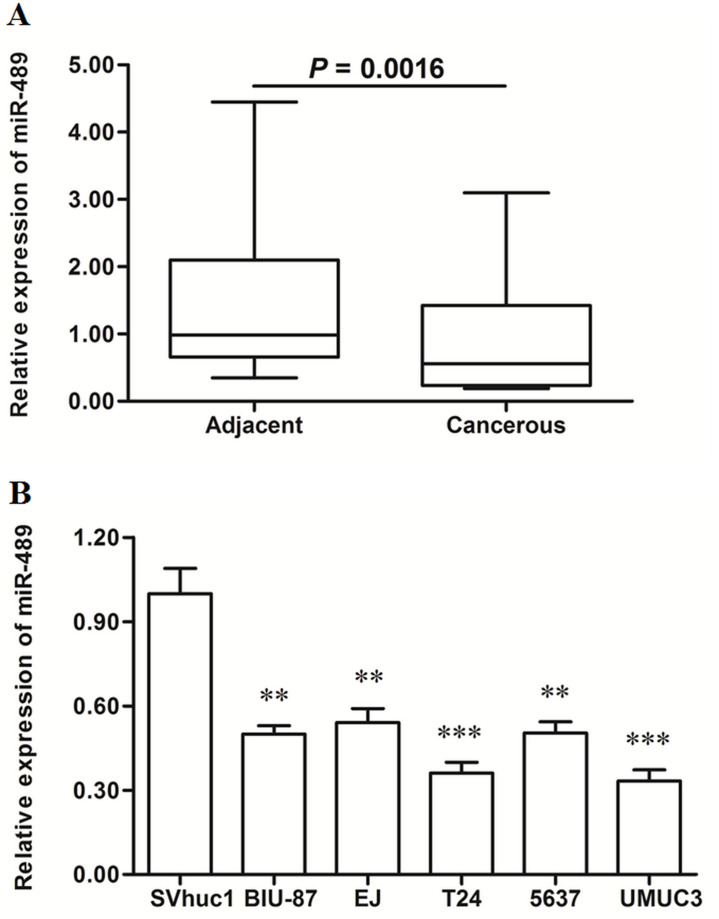
miR-489 Was Sharply Reduced in Human Bladder Cancer Tissues and Cell Lines
The expression levels were detected in bladder cancer tissues and matched adjacent normal tissues. Real-time qPCR analysis showed that miR-489 was reduced by about half in the cancerous tissues (p < 0.01) (Fig. 1A). Then the miR-489 levels in human normal bladder epithelial cell line and five bladder cancer cell lines were also detected. The results showed that miR-489 was reduced by more than 45% in all the cancer cell lines (p < 0.01), even by more than 60% in T24 and UMUC3 cells (p < 0.001) (Fig. 1B).
Figure 1.
miR-489 was significantly downregulated in human bladder cancer tissues and cell lines. Bladder cancer tissues and matched normal adjacent tissues were sampled from 48 bladder cancer patients. The expression levels of miR-489 were detected in these tissues with real-time quantitative polymerase chain reaction (qPCR). (A) miR-489 was significantly downregulated in bladder cancer tissues. The expression levels of miR-489 were detected in human normal bladder epithelial cell SV-HUC-1 and five types of human bladder cancer cell lines with real-time qPCR, including BIU-87, EJ, T24, 5637, and UMUC3. (B) miR-489 was significantly downregulated in bladder cancer cell lines. **p < 0.01, ***p < 0.001 versus SV-HUC-1.
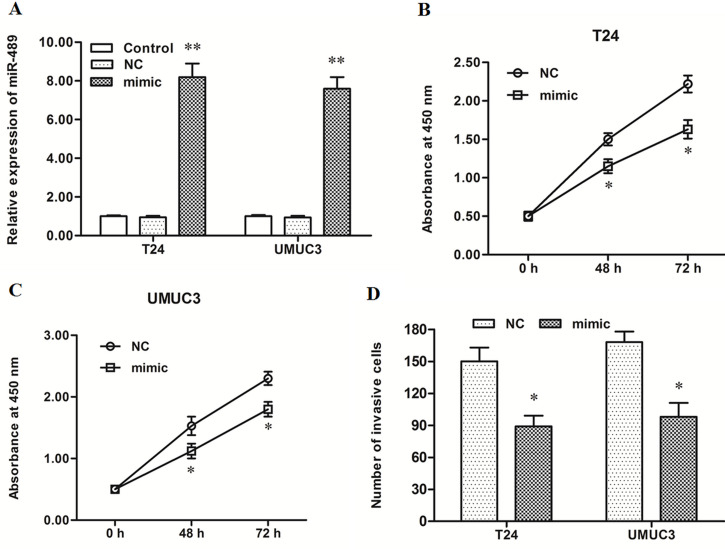
miR-489 Mimic Transfection Suppressed Proliferation and Invasion of Human Bladder Cancer Cells
The control mimic or miR-489 mimic was transfected into T24 and UMUC3 bladder cancer cells. After incubation for 48 h, miR-489 expression, cell proliferation, and cell invasion were detected. Real-time qPCR analysis showed that miR-489 expression was increased about eightfold by the miR-489 mimic transfection (p < 0.01) (Fig. 2A). CCK-8 and Transwell invasion assays showed that the miR-489 mimic suppressed the proliferation and invasion of T24 and UMUC3 bladder cancer cells (p < 0.05) (Fig. 2B–D).
Figure 2.
miR-489 mimic transfection suppressed proliferation and invasion of T24 and UMUC3 bladder cancer cell lines. The negative control mimic (NC) or miR-489 mimic was transfected into T24 and UMUC3 human bladder cancer cell lines. After incubation for 48 h, miR-489 expression was detected with real-time qPCR, cell proliferation was detected with the CCK-8 method, and cell invasion was detected with the Transwell invasion assay. (A) miR-489 expression was increased greatly by the miR-489 mimic transfection. (B, C) The miR-489 mimic significantly suppressed the proliferation of T24 and UMUC3 cells. (D) The miR-489 mimic significantly suppressed the invasion of T24 and UMUC3. *p < 0.05, *p < 0.01 versus NC.
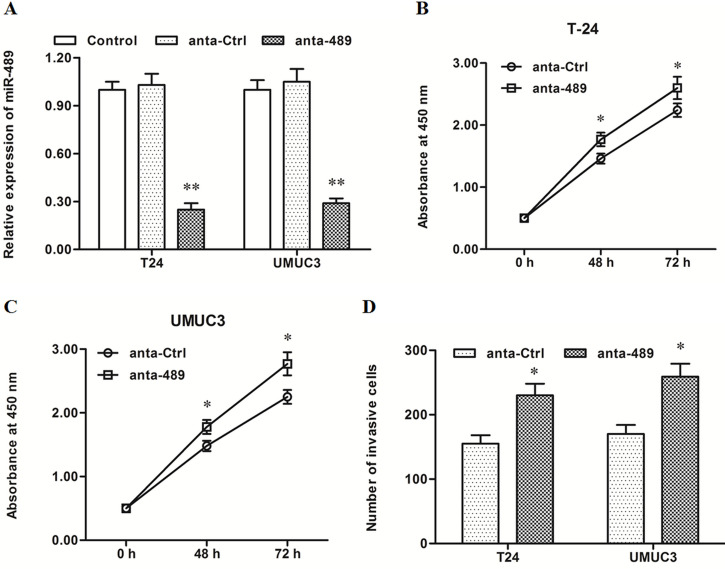
miR-489 Inhibitor Transfection Promoted Proliferation and Invasion of T24 and UMUC3 Bladder Cancer Cells
Control anta oligo or anti-miR-489 oligo was transfected into T24 and UMUC3 cells. After incubation for 48 h, miR-489 expression, cell proliferation, and cell invasion were detected. In contrast, the results from the miR-489 mimic transfection and miR-489 expression were sharply decreased by the miR-489 inhibitor transfection (p < 0.01) (Fig. 3A). The miR-489 inhibitor significantly promoted proliferation and invasion of T24 and UMUC3 cells (p < 0.05) (Fig. 3B–D). These data on the mimic and inhibitor transfection indicated that miR-489 had a negative effect on proliferation and invasion of bladder cancer cells.
Figure 3.
miR-489 silencing increased proliferation and invasion of T24 and UMUC3 cells. The control or anta-miR-489 oligo was transfected into T24 and UMUC3 cells. After incubation for 48 h, miR-489 expression, cell proliferation, and cell invasion were detected. (A) miR-489 level was sharply decreased by the anta-miR-489 transfection. (B, C) The anta-miR-489 significantly promoted the proliferation of T24 and UMUC3 cells. (D) The anta-miR-489 significantly promoted the invasion of T24 and UMUC3 cells. *p < 0.05, *p < 0.01 versus anta-Ctrl.
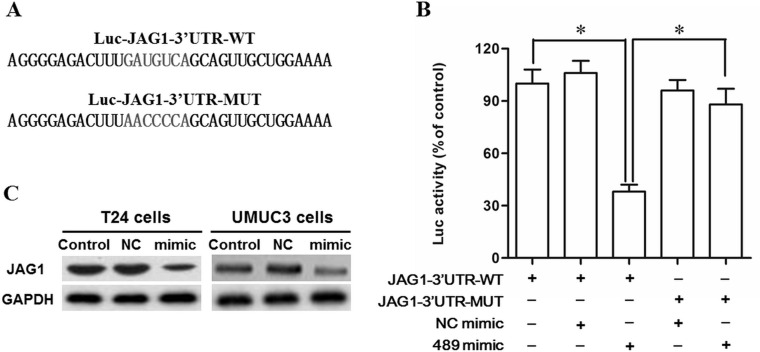
miR-489 Directly Targeted the JAG1 mRNA and Suppressed Expression of the JAG1 Protein
Bioinformatics analysis was used to assess the target relationship between miR-489 and JAG1. The sequence analysis showed that the 3′-UTR region of the JAG1 mRNA sequences was conserved in humans and mammals, and seven consecutive bases completely matched with the seed of miR-489 (Fig. 4A). The output of the TargetScan and miRDB online servers showed that miR-489 potentially targeted JAG1 mRNA at this region (Fig. 4B and C). WT and mutant 3′-UTR luciferase reporter gene check reporters were respectively constructed (Fig. 5A) and then transfected into the HEK293 cells with miR-489 mimic or control mimic. The data on luciferase activity detection revealed that miR-489 markedly weakened the luciferase activity in the WT group but not in the mutant group (Fig. 5B). To further validate that JAG1 was a target of miR-489, the control or miR-489 mimic was transfected into T24 and UMUC3 cells. Western blotting indicated that the JAG1 protein level was sharply reduced by the miR-489 mimic transfection (Fig. 5C). These data suggested that miR-489 suppressed proliferation and invasion of bladder cancer cells by directly targeting the tumor promoter JAG1.
Figure 4.
miR-489 potentially targeted Jagged1 (JAG1) mRNA at the 3′-untranslated region (3′-UTR) region. (A) Alignment of human miR-489 and JAG1 mRNA sequences among mammals. The outputs of the (B) TargetScan and (C) miRDB online servers on the binding of miR-489 and 3′-UTR region of JAG1 mRNA.
Figure 5.
miR-489 suppressed JAG1 expression by directly targeting JAG1. The NC mimic and miR-489 mimic were, respectively, cotransfected with a WT or mutant 3′-UTR dual luciferase vector into HEK293 cells. After incubation for 48 h, the luciferase activity in each group was measured using a microplate reader. (A) The WT and mutant 3′-UTR sequences at the miR-489 binding site. (B) miR-489 mimic suppressed the luciferase activity of the WT vector but not the mutant vector. The miR-489 mimic or inhibitor was transfected into T24 and UMUC3 cells. After incubation for 48 h, the JAG1 protein expression was detected with Western blotting. (C) miR-489 markedly suppressed the JAG1 protein expression. *p < 0.05.
DISCUSSION
miR-489 is a conserved miRNA that was identified and cloned in 2005 (19). From then on, researchers revealed that miR-489 played an important role in the regulation of several normal and cancerous tissue functions, including cardiac hypertrophy, maintenance of muscle stem cell quiescence, and tumor progression and resistance (20–22). Recently, miR-489 was proven to have an inhibitory role in several cancers (18,22,23). However, the role of miR-489 is not clear in the progression of other cancers. Here we report that miR-489 expression was sharply reduced in human bladder cancer tissues and cell lines, compared with normal adjacent tissues and normal bladder epithelial cells. Moreover, our data on miR-489 mimic and anta oligo transfection indicated that miR-489 had a suppressive effect on the proliferation and invasion of bladder cancer cells.
As is known, the role of miRNA mainly depends on its target gene. miR-489 targeted several proto-oncogenes and functioned as a tumor suppressor (16,22,24,25). For example, miR-489 targeted cell survival, promoting proto-oncogene Akt3 to increase cisplatin resistance of human ovarian cancer cells (22). In this study, our results from bioinformatics and luciferase reporter gene assays showed that miR-489 directly targeted the mRNA 3′-UTR region of JAG1, suggesting that miR-489 suppressed bladder cancer cell proliferation and invasion through targeting JAG1.
JAG1 was the first identified Notch ligand that interacts with four Notch receptors (26). The JAG1–Notch interaction could trigger a cascade of proteolytic cleavages to activate the transcription of multiple downstream target genes involved in the determination of cellular fate, tissue development, and cancer progression (27–29). A higher level of JAG1 has been observed in several human cancers and was associated with poor outcome (30–32). A dozen miRNAs were shown to target JAG1 to suppress tumor growth and drug resistance, such as microRNA-199b-5p, miR-200c, and miR-141 (33,34). To date, little information has been revealed on the role of JAG1 in bladder cancer genesis. A recent study showed that inactivated mutation of JAG1 was shown to have a suppressive effect on tumor invasion in bladder cancer (35). In this study, our results also suggested that suppression of JAG1 might be associated with suppression of bladder cancer cell progression and invasion.
In conclusion, miR-489 expression was reduced in human bladder cancer tissues and cell lines. miR-489 had a suppressive effect on the proliferation and invasion of bladder cancer cells.
REFERENCES
- 1. Burger M.; Catto J. W.; Dalbagni G.; Grossman H. B.; Herr H.; Karakiewicz P.; Kassouf W.; Kiemeney L. A.; La Vecchia C.; Shariat S. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 63(2):234–241; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Boormans J. L.; Zwarthoff E. C. Limited funds for bladder cancer research and what can we do about it. Bladder Cancer 2(1):49–51; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capaccione K. M.; Pine S. R. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis 34(7):1420–1430; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopan R.; Ilagan M. X. G. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 137(2):216–233; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehebauer M.; Hayward P.; Martinez-Arias A. Notch signaling pathway. Sci. STKE 2006(364):cm7; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Artavanis-Tsakonas S.; Rand M. D.; Lake R. J. Notch signaling: Cell fate control and signal integration in development. Science 284(5415):770–776; 1999. [DOI] [PubMed] [Google Scholar]
- 7. Fortini M. E. Notch signaling: The core pathway and its posttranslational regulation. Dev. Cell 16(5):633–647; 2009. [DOI] [PubMed] [Google Scholar]
- 8. Borggrefe T.; Oswald F. The Notch signaling pathway: Transcriptional regulation at Notch target genes. Cell Mol. Life Sci. 66(10):1631–1646; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takebe N.; Nguyen D.; Yang S. X. Targeting notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol. Ther. 141(2):140–149; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu Y.-Y.; Zheng M.-h.; Zhang R.; Liang Y.-M.; Han H. Notch signaling pathway and cancer metastasis. In: Reichrath J.; Reichrath S., eds. Notch signaling in embryology and cancer. New York: Springer; 2012:186–198. [DOI] [PubMed] [Google Scholar]
- 11. Bartel D. P. MicroRNAs: Target recognition and regulatory functions. Cell 136(2):215–233; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niemitz E. MicroRNAs dampen noisy expression. Nat. Genet. 47(5):429–429; 2015. [Google Scholar]
- 13. Grimson A. Noncoding RNA: Linking microRNAs to their targets. Nat. Chem. Biol. 11(2):100–101; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Lin S.; Gregory R. I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 15(6):321–333; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zimmerman A. L.; Wu S. MicroRNAs, cancer and cancer stem cells. Cancer Lett. 300(1):10–19; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie Z.; Cai L.; Li R.; Zheng J.; Wu H.; Yang X.; Li H.; Wang Z. Down-regulation of miR-489 contributes into NSCLC cell invasion through targeting SUZ12. Tumor Biol. 36(8):6497–6505; 2015. [DOI] [PubMed] [Google Scholar]
- 17. Patel Y.; Shah N.; Botbyl R.; Lee J.; Chen H. miR-489 function as tumor suppressor miRNA by targeting HER2 in HER2-positive breast cancer. Cancer Res. 75(15 Suppl.):3120–3120; 2015. [Google Scholar]
- 18. Kikkawa N.; Hanazawa T.; Fujimura L.; Nohata N.; Suzuki H.; Chazono H.; Sakurai D.; Horiguchi S.; Okamoto Y.; Seki N. miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC). Br. J. Cancer 103(6):877–884; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bentwich I.; Avniel A.; Karov Y.; Aharonov R.; Gilad S.; Barad O.; Barzilai A.; Einat P.; Einav U.; Meiri E. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 37(7):766–770; 2005. [DOI] [PubMed] [Google Scholar]
- 20. Cheung T. H.; Quach N. L.; Charville G. W.; Liu L.; Park L.; Edalati A.; Yoo B.; Hoang P.; Rando T. A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482(7386):524–528; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang K.; Liu F.; Zhou L.-Y.; Long B.; Yuan S.-M.; Wang Y.; Liu C.-Y.; Sun T.; Zhang X.-J.; Li P.-F. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 114(9):1377–1388; 2014. [DOI] [PubMed] [Google Scholar]
- 22. Wu H.; Xiao Z.; Zhang H.; Wang K.; Liu W.; Hao Q. miR-489 modulates cisplatin resistance in human ovarian cancer cells by targeting Akt3. Anticancer Drugs 25(7):799–809; 2014. [DOI] [PubMed] [Google Scholar]
- 23. Jiang L.; He D.; Yang D.; Chen Z.; Pan Q.; Mao A.; Cai Y.; Li X.; Xing H.; Shi M. miR-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett. 588(11):2009–2015; 2014. [DOI] [PubMed] [Google Scholar]
- 24. Patel Y.; Shah N.; Lee J. S.; Markoutsa E.; Jie C.; Liu S.; Botbyl R.; Reisman D.; Xu P.; Chen H. A novel double-negative feedback loop between miR-489 and the HER2-SHP2-MAPK signaling axis regulates breast cancer cell proliferation and tumor growth. Oncotarget 7(14):18293–18308; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X.; Wang Y. W.; Xing A. Y.; Xiang S.; Shi D. B.; Liu L.; Li Y. X.; Gao P. Suppression of SPIN1-mediated PI3K/Akt pathway by miR-489 increases chemosensitivity in breast cancer. J. Pathol.; 2016. [DOI] [PubMed] [Google Scholar]
- 26. Sethi N.; Dai X.; Winter C. G.; Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19(2):192–205; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiernan A. E.; Xu J.; Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2(1):e4; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grochowski C. M.; Loomes K. M.; Spinner N. B. Jagged1 (JAG1): Structure, expression, and disease associations. Gene 576(1):381–384; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katoh M.; Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 17(4):681–685; 2006. [PubMed] [Google Scholar]
- 30. Sun W.; Gaykalova D. A.; Ochs M. F.; Mambo E.; Arnaoutakis D.; Liu Y.; Loyo M.; Agrawal N.; Howard J.; Li R. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 74(4):1091–1104; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reedijk M.; Odorcic S.; Chang L.; Zhang H.; Miller N.; McCready D. R.; Lockwood G.; Egan S. E. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 65(18):8530–8537; 2005. [DOI] [PubMed] [Google Scholar]
- 32. Dickson B. C.; Mulligan A. M.; Zhang H.; Lockwood G.; O’Malley F. P.; Egan S. E.; Reedijk M. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod. Pathol. 20(6):685–693; 2007. [DOI] [PubMed] [Google Scholar]
- 33. Liu M. X.; Siu M. K.; Liu S. S.; Yam J. W.; Ngan H. Y.; Chan D. W. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget 5(4):944; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallejo D. M.; Caparros E.; Dominguez M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 30(4):756–769; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rampias T.; Vgenopoulou P.; Avgeris M.; Polyzos A.; Stravodimos K.; Valavanis C.; Scorilas A.; Klinakis A. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 20(10):1199–1205; 2014. [DOI] [PubMed] [Google Scholar]