Abstract
It has been demonstrated that microRNAs (miRNAs) act as oncogenes or tumor suppressors in a variety of cancers. Our previous work suggested that miR-10a/b functioned as a tumor suppressor in gastric cancer, and miR-10b was also reported to be significantly downregulated in advanced stage cervical cancer tissues. However, the aberrant expression of miR-10b in cervical cancer and its possible role in cervical carcinogenesis was largely unknown. In this study, we investigated the expression of miR-10b in cervical cancer tissues, carcinoma in situ tissues, mild dysplasia, moderate dysplasia, severe dysplasia tissues, and normal controls. We found that miR-10b was significantly downregulated during cervical cancer progression, and the lower level of miR-10b in cervical cancer was significantly associated with a more aggressive tumor phenotype. Moreover, overexpression of miR-10b in cervical cancer cells could inhibit the cell proliferation and invasion, and the further mechanism study suggested that its role was possibly through directly targeting HOXA1. These results suggested that the downregulation of miR-10b and the resulting elevated HOXA1 level in cervical cancer tissues might play critical roles in cervical cancer progression.
Key words: Cervical cancer, miR-10b, HOXA1, Tumor suppressor
INTRODUCTION
Cervical cancer is the second most common gynecological tumor in the world. Approximately 500,000 cases of cervical cancer are diagnosed per year, about 45% of which result in death (1,2). Cervical cancer will progress through mild dysplasia, moderate dysplasia, severe dysplasia, carcinoma in situ, and infiltrating carcinoma processes from normal tissue to cervical cancer. This is a complex progression involving the abnormal expression of many oncogenes and tumor-suppressor genes. Current treatment approaches for cervical cancer include surgery, radiotherapy, and chemotherapy (3). However, how early the cancer is detected is most critical for therapy outcomes. So, to discover and identify new biomarkers for earlier stages of cervical cancer is urgently required for earlier detection of cervical cancer and individualized therapies. Recently, the roles of microRNAs (miRNAs) as potential biomarkers and therapy targets have been widely investigated in diverse cancers.
miRNAs are endogenous small noncoding RNA molecules that function in many biologic processes through negatively regulating target mRNAs via resulting mRNA cleavage or repressing mRNA translation. It has been demonstrated that miRNAs play a regulatory role in diverse biological processes, and the aberrant expression of miRNAs is found in many diseases, especially in cancer (4). miRNAs have been reported to act as oncogenes or tumor suppressors in a variety of cancers, such as breast, pancreatic, lung, hepatic cancer, and gastric cancer (5–10). Currently, the aberrant expression of many miRNAs has been observed in cervical cancer. For example, miR-21, miR-142-3p, miR-155, miR-34a, and let-7a have been shown to contribute to cervical carcinogenesis by changing the cell cycle, cell apoptosis, cell migration, and invasion through targeting the relative genes (11–15). In addition, a number of miRNAs have been shown to be associated with different stages and might be developed as potential biomarkers for cervical cancer diagnosis (16). Therefore, exploring the aberrant expression pattern of miRNAs and the role of miRNAs in cervical cancer will be meaningful in understanding the mechanism of cervical carcinogenesis and also for developing novel diagnosis and therapy methods for cervical cancer.
Our previous work suggested that miR-10a/b might function as a tumor suppressor in gastric cancer and was regulated by DNA methylation (17,18). miR-10b was also reported to be significantly downregulated in advanced stage small-cell carcinoma of the cervix (SCCC) patients compared to early stage SCCC patients (19). However, the aberrant expression of miR-10b in cervical cancer and its possible role in cervical carcinogenesis were largely unknown.
In our reports, we accurately measured the expression of miR-10b in cervical cancer tissues, carcinoma in situ tissues, mild dysplasia, moderate dysplasia, severe dysplasia tissues, and normal controls through TaqMan probe-based real-time PCR. The results showed that miR-10b was obviously downregulated during cervical cancer progression, and a more aggressive tumor phenotype was clearly associated with the lower expression of miR-10b in cervical cancer. Overexpression of miR-10b in cervical cancer cells also could inhibit the cell proliferation rate and the ability of invasion, and the mechanism study showed that the tumor-suppressive role of miR-10b was possibly by targeting HOXA1.
MATERIALS AND METHODS
Patients and Specimens
The human clinical samples were collected from surgical specimens at Chongqing Cancer Hospital. The study was approved by the ethical board of the hospital. All samples were immediately snap frozen in liquid nitrogen and then stored at −80°C until RNA extraction.
Cell Culture and Transfections
The human cervical cancer cell lines, Hela and SiHa cells, were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in 5% CO2. 293T cells were also cultured in the above medium. miR-10b mimics, miR-10b inhibitors, HOXA1 siRNA, and scramble control were ordered from Dharmacon and transfected into Hela or SiHa cells at a final concentration of 50 nM with DharmFECT1.
RNA Extraction, cDNA Synthesis, and Real-Time PCR Assays
Total RNA was extracted using TRIzol from tissues and cells according to the manufacturer’s instructions. RNA was quantified by absorbance at 260 nm, and cDNA was synthesized by M-MLV reverse transcriptase from 2 µg of the total RNA. For detection of miR-10b expression, a miR-10b-specific stem-loop RT primer was used for the reverse transcription. Quantitative RT-PCR was performed on a Bio-Rad CFX96 real-time PCR System with TaqMan probes. The PCR conditions were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 34 s. The expression of miR-142-3p was normalized by the endogenous U6 snRNA. The sequences of primers are presented in Table 1.
Table 1.
Sequence of Primers Used in qRT-PCR
| Primer | Sequence (5′→3′) |
|---|---|
| miR-10b RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGCACAAATT |
| miR-10b forward | AGCTGTTCAGTGCACTACAGA |
| miR-10b reverse | GTGCTACCCTGTAGAAC |
| miR-10b probe | FAM-CCTGTAGAACCGAATTTG-MGB |
| U6 RT | AAAATATGGAACGCTTCACGAATTTG |
| U6 forward | CTCGCTTCGGCAGCACATATACT |
| U6 reverse | ACGCTTCACGAATTTGCGTGTC |
| U6 probe | FAM-CCATGCTAATCTTCTCTGTA-MGB |
Cell Proliferation Assay
To examine the effect of miR-10b mimics or inhibitors on cell proliferation rate, the transfected cells were incubated with 10% CCK-8 (DOJINDO) at 37°C until visual color conversion appeared and then tested at 450 nm absorption. Proliferation rates were detected at days 1, 2, 3, and 4 posttransfection, and quantification was done on a microtiter plate reader according to the manufacturer’s protocol.
Cell Invasion Assays
Hela and SiHa cells were seeded onto a Matrigel-coated chamber inserting into a well of a 24-well plate in serum-free medium. FBS was added to the well below the chamber as a chemoattractant. After 24 h, invasive cells located on the lower surface of the chamber were stained with 0.1% crystal violet and counted.
Constructs and Luciferase Assay
The 3′-UTR of the human HOXA1 containing the miR-10b binding site was PCR amplified and cloned into the pMIR-reporter downstream of the firefly luciferase gene. Mutations at the miRNA binding site in the 3′-UTR sequences were created using bridging PCR, and then the mutated HOXA1 3′-UTR was also cloned into the pMIR-reporter to generate the mutated one. For luciferase assay, 293T cells were cotransfected with 0.4 µg of the reporter construct, 0.02 µg of pRL-TK vector, and 5 pmol of miRNA mimic or scramble controls. Cells were harvested 48 h posttransfection and detected with Dual Luciferase Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. All transfection assays were carried out in triplicate.
Western Blotting
The whole-cell lysate of Hela and SiHa cells was extracted using lysis buffer (0.05 M Tris, pH 7.5, 0.15 M NaCl, 2% NP-40) containing 200 mM of Na3VO4, 200 mM of NaF, 0.5 M of EDTA, and proteinase inhibitors for 30 min on ice and then quantified by BCA protein assay kit. Proteins (20 µg) were separated by SDS-PAGE and then were transferred onto a PVDF membrane. The following antibodies were used for Western blot: GAPDH (10494-1-AP; Proteintech) and HOXA1 (13513-1-AP; Proteintech).
Statistics
Student’s t-test (two tailed) was performed to analyze the data. Values of p < 0.05 were considered significantly.
RESULTS
miR-10b Is Significantly Downregulated in Cervical Cancer
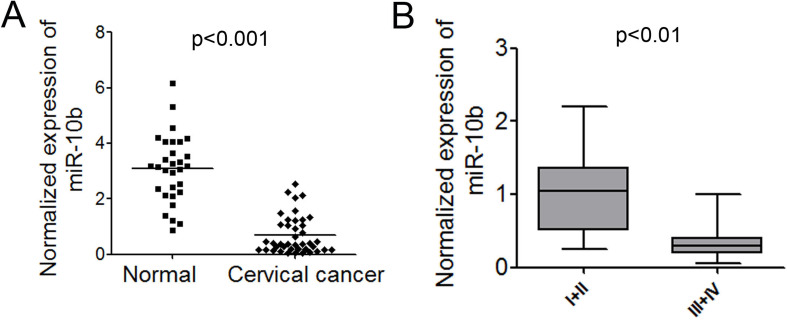
TaqMan probe-based qPCR was employed to accurately measure the expression levels of miR-10b in cervical cancer. We first examined the expression of miR-10b in 40 cervical cancer tissue samples and 30 normal controls. The expression level of miR-10b in most of the cervical cancer tissues was significantly lower than that in normal controls (p < 0.001) (Fig. 1A). To further investigate the correlation between the expression of miR-10b and the clinicopathological characteristics, the relative expression of miR-10b in 40 cervical cancer patients was statistically analyzed. Correlation analysis showed that lower-level expression of miR-10b in cervical cancer was associated with a more aggressive tumor phenotype (p < 0.01, stage I/II vs. III/IV) (Fig. 1B). However, no significant associations with gender, age, or Borrmann typing were observed. The correlation of lower levels of miR-10b with the more aggressive phenotype of cervical cancer strongly suggested that miR-10b might play an important role in cervical carcinogenesis.
Figure 1.
miR-10b is significantly downregulated in cervical cancer tissues. (A) Normalized expression of miR-10b in 40 cervical cancer tissues and 30 normal controls. (B) The correlation of miR-10b expression with tumor stages.
miR-10b Decreased During Cervical Cancer Progression and its Lower Expression Had Positive Correlation With Malignant Transformation
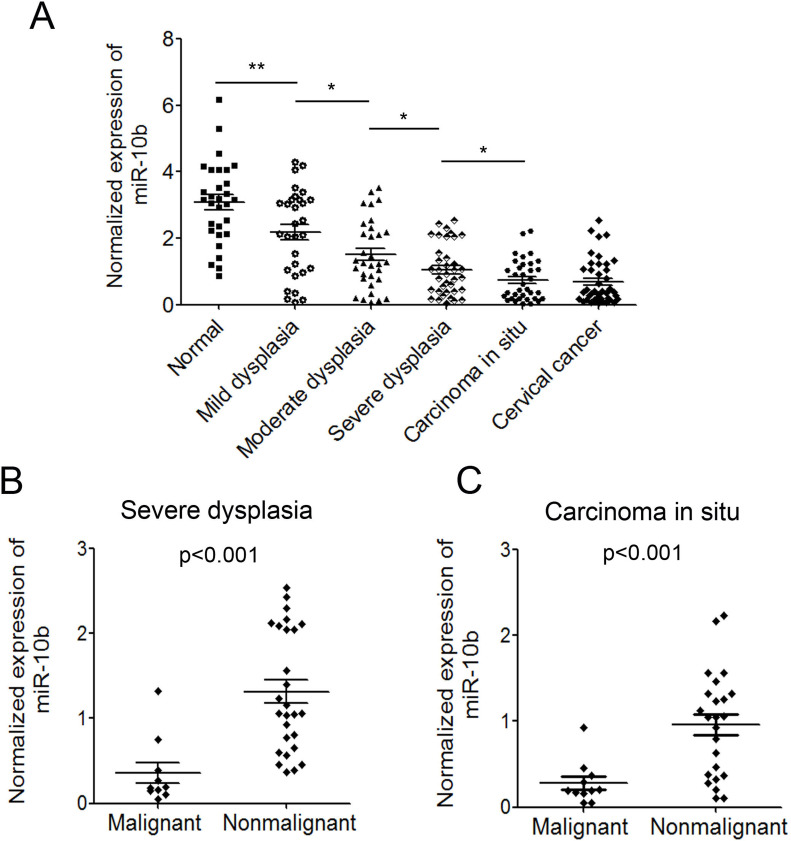
The progression of cervical cancer undergoes several different phases: dysplasia (including mild dysplasia, moderate dysplasia, and severe dysplasia), carcinoma in situ (CIS), and cervical cancer. To further investigate the possible role of miR-10b in cervical cancer progression, we measured the levels of miR-10b in the different phases. We examined the expression of miR-10b in 30 mild dysplasias, 32 moderate dysplasias, 37 severe dysplasias, and 35 CIS tissues. We also compared the expression level with that in normal controls and cervical cancer tissues, described above. The results showed that the expression level of miR-10b in mild dysplasia tissues was significantly lower than that in normal tissues, and the level of miR-10b in moderate dysplasia tissues was even lower than that in mild dysplasia tissues. The expression of miR-10b decreased gradually during cervical cancer progression (Fig. 2A). In addition, we analyzed the expression of miR-10b in the dysplasia tissues of few malignant transformed patients. The average expression level of miR-10b in the dysplasia tissues of 10 malignant transformed patients was significantly lower than that in the currently nonmalignant transformed patients (p < 0.001) (Fig. 2B). The expression level of miR-10b in CIS tissues of 11 malignant transformed patients was also obviously lower than that in the currently nonmalignant transformed patients (p < 0.001) (Fig. 2C). These results suggested that patients with a lower expression level of miR-10b might be more prone to malignant transformation. The correlation of the expression level of miR-10b and malignant transformation will be investigated in more clinical samples.
Figure 2.
miR-10b decreased during cervical cancer progression and its correlation with malignant transformation. (A) Normalized expression of miR-10b in several different phases during progression of cervical cancer. (B) The correlation of miR-10b expression in dysplasia tissues with malignant transformation. (C) The correlation of miR-10b expression in CIS tissues with malignant transformation. Data are shown as mean ± SD. *p < 0.05; **p < 0.01.
Reintroduction of miR-10b in Cervical Cancer Cells Can Inhibit Cell Growth and Invasion
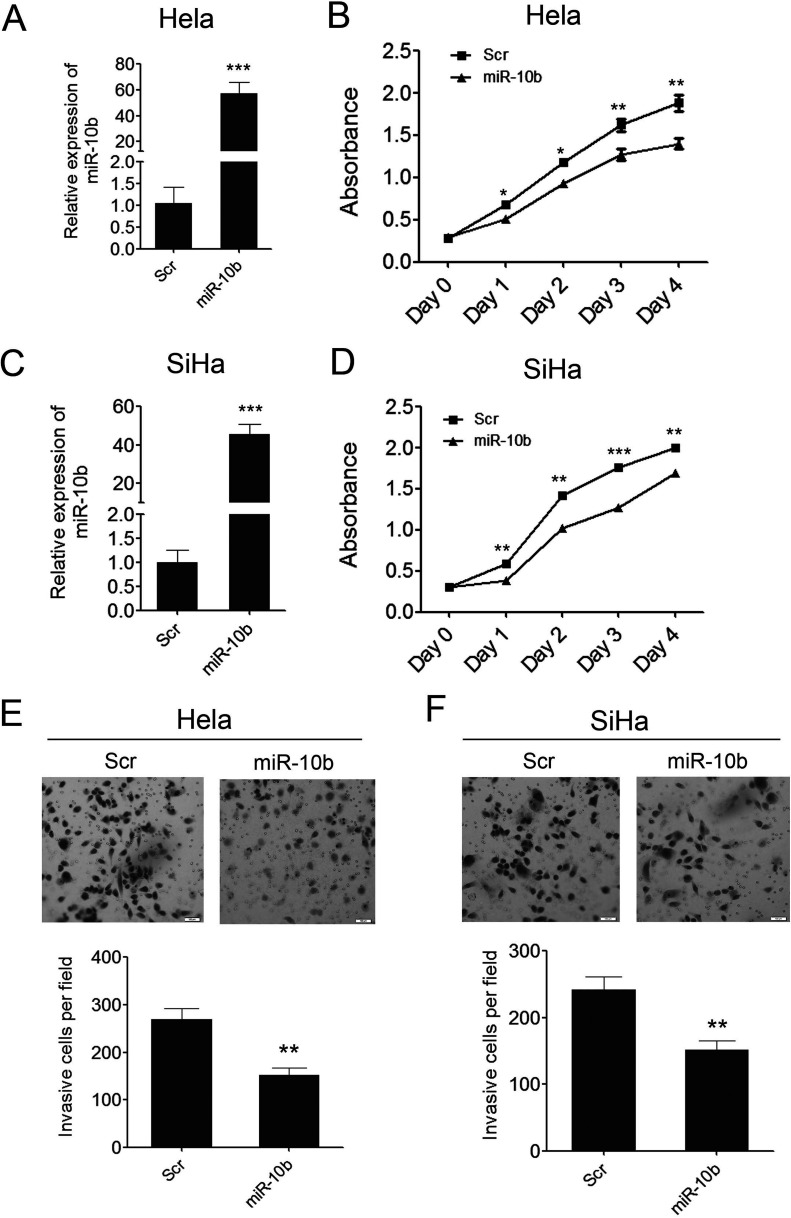
To investigate the role of miR-10b in cervical carcinogenesis, we reintroduced miR-10b mimics into two cervical cancer cell lines, Hela and SiHa. miR-10b was successfully overexpressed in these two cell lines, which was confirmed by qPCR. As shown in Figure 3A and C, miR-10b was overexpressed by 60-fold and 50-fold, relative to the scrambled control, in Hela and SiHa cells respectively. As a result, the overexpression of miR-10b in both of the cervical cancer cell lines can inhibit cell proliferation significantly as demonstrated by CCK-8 growth assay (Figs. 3B, D). We further assessed the effects of miR-10b on cell invasion, which were the important determinants of malignant progression and metastasis. We conducted a Matrigel cell invasion assay to assess the role of miR-10b in regulating cell invasion. The Matrigel cell invasion assay showed that overexpression of miR-10b in the two cervical cancer cell lines dramatically decreased the invaded cells compared with the scramble control (Figs. 3E, F). These results indicated that miR-10b played important roles in cell proliferation and invasiveness in cervical carcinogenesis as a tumor suppressor.
Figure 3.
miR-10b can inhibit cervical cancer cell growth and invasion. (A) Enforced expression of miR-10b in Hela cells was validated by qRT-PCR. (B) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of Hela cells. (C) Enforced expression of miR-10b in SiHa cells was validated by qRT-PCR. (D) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of SiHa cells. (E) The invaded Hela cells in the Matrigel Transwell invasion assay. (F) The invaded SiHa cells in the Matrigel Transwell invasion assay. Data are shown as mean ± SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001.
Inhibition of miR-10b in Cervical Cancer Cells Can Promote Cell Growth and Invasion
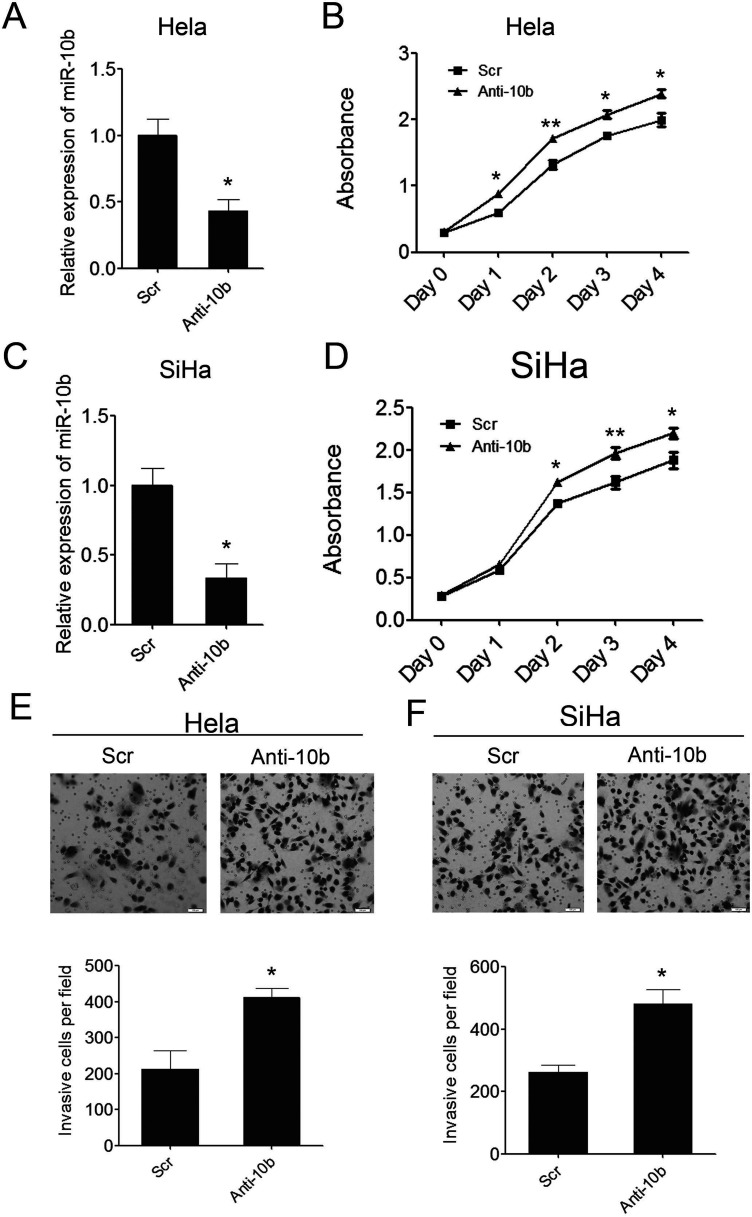
To further determine the tumor suppressor role of miR-10b in cervical carcinogenesis, we introduced miR-10b inhibitors into the above two cervical cancer cell lines to suppress the endogenous miR-10b expression. miR-10b was successfully suppressed in these two cell lines, which was confirmed by q-PCR (Fig. 4A, C). As a result, the suppression of miR-10b in both of the two cervical cancer cell lines can promote cell proliferation significantly as demonstrated by CCK-8 growth assay (Fig. 4B, D). The Matrigel cell invasion assay showed that suppression of miR-10b in the two cervical cancer cell lines dramatically increased the invaded cells compared with the scramble control (Fig. 4E, F). These results demonstrated that miR-10b functioned as a tumor suppressor in cervical carcinogenesis and that downregulation of miR-10b might demonstrate risk of cervical carcinogenesis.
Figure 4.
Inhibition of miR-10b in cervical cancer cells can promote cell growth and invasion. (A) Inhibition of miR-10b in Hela cells was confirmed by qRT-PCR. (B) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of Hela cells. (C) Inhibition of miR-10b in SiHa cells was confirmed by qRT-PCR. (D) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of SiHa cells. (E) The invaded Hela cells in the Matrigel Transwell invasion assay. (F) The invaded SiHa cells in the Matrigel Transwell invasion assay. Data are shown as mean ± SD (n = 3). *p < 0.05; **p < 0.01.
miR-10b Targets HOXA1 in Cervical Cancer Cells
We further dissected the mechanism by which miR-10b functioned as a tumor suppressor in cervical cancer. miR-10a has been reported to directly target the oncogenic HOXA1 gene in pancreatic cancer and gastric cancer (17,20). Home homeobox (HOX) genes are highly conserved transcription factors that are a determinant for correct anterior–posterior patterning of the body axis. Knockdown of HOXA1 can suppress gastric cancer cell growth and migration. Since miR-10b had the same seed sequence as miR-10a, we asked whether miR-10b regulated cervical cancer progression by targeting HOXA1.
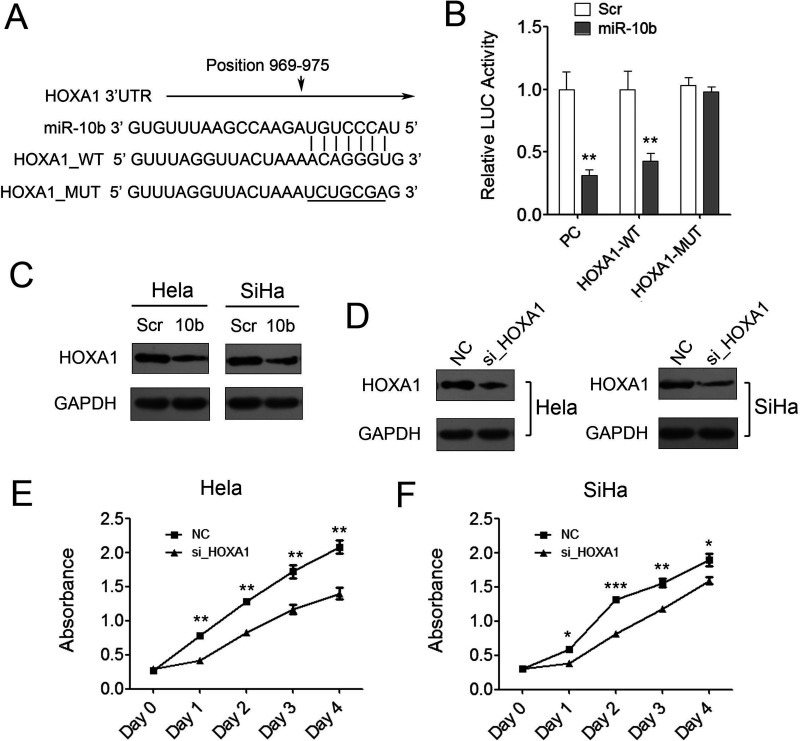
miR-10b was predicted to bind to the 3′-UTR region of HOXA1 using two algorithms PicTar and TargetScan (Fig. 5A). To validate the negative regulation of miR-10b on HOXA1, we cloned the 3′-UTR of HOXA1 into a luciferase reporter construct (pMIR-reporter) meanwhile cloned nucleotide sequence complete complementary to miR-10b as positive control (PC). A dramatic decrease of luciferase activity of PC demonstrated that miR-10b was successfully expressed in 293T cells, and the reporter assay was convincing. The results also showed that miR-10b mimics repressed the luciferase activity of HOXA1 3′-UTR significantly compared with the scramble control (Fig. 5B). To further examine whether the repression was dependent on the miRNA binding site, another luciferase reporter containing miRNA binding site mutated 3′-UTR of HOXA1 was constructed. The results indicated that mutation of the miRNA binding sites in the HOXA1 3′-UTR abrogated the reduction in luciferase activities, which suggested that the repression of miR-10b on HOXA1 3′-UTR was dependent on the miRNA binding site (Fig. 5B). Consistent with the reporter assay, we observed an evident decrease in HOXA1 protein in the presence of miR-10b mimics compared to scramble control in both of Hela and SiHa cells (Fig. 5C).
Figure 5.
HOXA1 is a direct target of miR-10b in cervical cancer cells. (A) The prediction of the binding between miR-10b and HOXA1 by Pictar. (B) Relative luciferase activity of the indicated reporter constructs in 293T cells cotransfected with miR-10b mimics or control. (C) Western blot showed HOXA1 protein expression in Hela and SiHa cells transfected with scramble control or miR-10b mimics. (D) Western blot showed HOXA1 protein expression in Hela and SiHa cells transfected with negative control or HOXA1 siRNAs. (E, F) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of Hela and SiHa cells. Data are shown as mean ± SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001.
Knockdown of HOXA1 Can Inhibit Cervical Cancer Cell Growth
To investigate the role of HOXA1 in cervical cancer progression, we knocked down HOXA1 expression using siRNAs in two cervical cancer cell lines. As shown in Figure 5D, the expression of HOXA1 was successfully knocked down in both of the two cell lines, which was confirmed by Western blot. Knockdown of HOXA1 attenuated cervical cancer cell proliferation significantly as demonstrated by CCK-8 growth assay compared with the negative control (Fig. 5E, F). These results indicated that HOXA1 might play an important role in cervical cancer as an oncogene. The aberrant downregulation of miR-10b in cervical cancer cell can give rise to upregulation of HOXA1 and further to promote cervical cancer progression. miR-10b also can be developed as a potential predictive biomarker for early diagnosis of cervical cancer as it has a significant correlation with malignant transformation.
DISCUSSION
Several studies have investigated the association between aberrant miR-10b expression and the risk of diverse cancers, but the results are inconsistent. Accumulating studies suggest that miR-10b may act as novel oncogene in human cancers (21–23). There are some contrary conclusions about the role of miR-10b in tumor progression. miR-10b was also reported to act as a tumor-suppressive gene in gastric carcinogenesis (24). The researchers found that miR-10b was silenced in gastric cancer cells by promoter methylation, and gastric cancer cells with an overexpression of miR-10b showed a significant decrease in colony formation and proliferation rates. The clinical study also indicated that miR-10b was significantly downregulated in gastric cancer tissues (18). However, the aberrant expression and the potential role of miR-10b in cervical cancer were largely unknown except that miR-10b was reported to be significantly downregulated in advanced stage SCCC patients (19).
In this study, we examined the expression of miR-10b in a number of patients with cervical dysplasia or cervical cancer and investigated the potential application of its expression level in cervical cancer diagnosis. The results indicated that miR-10b gradually downregulated during cervical cancer progression. Furthermore, the lower expression level of miR-10b was associated with more aggressive tumor phenotypes. Enforced expression of miR-10b in cervical cancer cells can clearly inhibit cervical cancer cell proliferation and migration. These results suggested that miR-10b might act as a tumor suppressor in cervical cancer. The downregulation of miR-10b would give rise to abnormal cervical cell proliferation and then the accumulation of aberrant cell proliferation, together with further drop of miR-10b, might lead to cervical cancer. However, the mechanism of miR-10b downregulating in cervical cancer was unknown. miR-10b had been reported to be regulated by DNA methylation, and its downregulation in gastric cancer was due to the hypermethylation of CpG islands upstream of miR-10b (18). We will further examine the methylation level of CpG islands upstream of miR-10b in cervical cancer tissues and uncover the mechanism of its aberrant expression in cervical cancer.
On the other hand, the downregulation of miR-10b during cervical cancer progression also suggested that miR-10b might serve as a potential biomarker in the early diagnosis of cervical cancer. Several innate properties of miRNAs, including small size, stability against degradation, and easy detectability, make them attractive as potential biomarkers. miRNAs are also detectable in bodily fluids including plasma, serum, urine, saliva, and tears (25,26). Furthermore, increasing evidence suggests that expression profiles of miRNAs in the plasma and/or serum of cancer patients can reflect the expression change of miRNAs in tumor cells (27). Circulating miRNAs will be a novel class of noninvasive biomarkers for cancer diagnostic and prognostic information. So the expression level of miR-10b in the serum of cervical cancer patients needs to be examined further to investigate the potential application of circulating miRNAs in cervical cancer diagnosis.
HOXA1 had been identified as a direct target of miR-10a in regulating several biological process (17,20,28). In this study, we showed that miR-10b also targeted HOXA1 to act in a tumor-suppressor role in cervical cancer progression. HOX genes encode a set of master transcription factors, which control pattern formation, differentiation, and proliferation during embryonic development. In humans, there are 39 class I HOX genes, which spread in four different clusters (A, B, C, and D) located on four chromosomes (7, 17, 12, and 2). An association between the deregulation of HOX gene expression and oncogenic transformation has been recently reported in human tumors. Hung et al. reported that HOXA1 was expressed only in cervical carcinoma cell lines and not in normal cervical tissues (29). Other reports also indicated that HOXA1 might be involved in cervical tumorigenesis (30). In our study, we indicated that HOXA1 played an oncogenic role in cervical cancer and knockdown of HOXA1 can inhibit cervical cancer cell growth. We also demonstrated that HOXA1 was a direct target of miR-10b in cervical cancer cells. So we concluded that miR-10b suppressed cervical cancer cell proliferation and invasion by targeting oncogenic HOXA1. The results also suggested that the downregulation of miR-10b and the resulting elevated HOXA1 level played a critical role in cervical carcinogenesis.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81171365) and Chongqing Municipal Science and Technology Commission (cstc2015jcsf10007). S.-L.L. conceived the project; D.-L.Z. designed the experiments and carried out the majority of the experiments; Q.Z. and D.W. helped to collect clinical samples; all authors discussed the results; D.-L.Z. and L.-L.G wrote the manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Arbyn M.; Castellsague X.; de Sanjose S.; Bruni L.; Saraiya M.; Bray F.; Ferlay J. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 22:2675–2686; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J.; Shin H. R.; Bray F.; Forman D.; Mathers C.; Parkin D. M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127:2893–2917; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Duenas-Gonzalez A.; Cetina L.; Mariscal I.; de la Garza J. Modern management of locally advanced cervical carcinoma. Cancer Treat. Rev. 29:389–399; 2003. [DOI] [PubMed] [Google Scholar]
- 4. Wu W. K.; Lee C. W.; Cho C. H.; Fan D.; Wu K.; Yu J.; Sung J. J. MicroRNA dysregulation in gastric cancer: A new player enters the game. Oncogene 29:5761–5771; 2010. [DOI] [PubMed] [Google Scholar]
- 5. Li Y.; Fang Y.; Liu Y.; Yang X. MicroRNAs in ovarian function and disorders. J. Ovarian Res. 8:51; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu D.; Chen H.; Yang X.; Chen W.; Wang L.; Xu J.; Yu L. miR-32 functions as a tumor suppressor and directly targets SOX9 in human non-small cell lung cancer. Onco. Targets Ther. 8:1773–1783; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Lee E. J.; Gusev Y.; Jiang J.; Nuovo G. J.; Lerner M. R.; Frankel W. L.; Morgan D. L.; Postier R. G.; Brackett D. J.; Schmittgen T. D. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer 120:1046–1054; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murakami Y.; Yasuda T.; Saigo K.; Urashima T.; Toyoda H.; Okanoue T.; Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25:2537–2545; 2006. [DOI] [PubMed] [Google Scholar]
- 9. Yu S.; Lu Z.; Liu C.; Meng Y.; Ma Y.; Zhao W.; Liu J.; Yu J.; Chen J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 70:6015–6025; 2010. [DOI] [PubMed] [Google Scholar]
- 10. Liu C.; Yu J.; Yu S.; Lavker R. M.; Cai L.; Liu W.; Yang K.; He X.; Chen S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J. Hepatol. 53:98–107; 2010. [DOI] [PubMed] [Google Scholar]
- 11. Deng B.; Zhang Y.; Zhang S.; Wen F.; Miao Y.; Guo K. MicroRNA-142-3p inhibits cell proliferation and invasion of cervical cancer cells by targeting FZD7. Tumour Biol. 36:8065–8073; 2015. [DOI] [PubMed] [Google Scholar]
- 12. Lao G.; Liu P.; Wu Q.; Zhang W.; Liu Y.; Yang L.; Ma C. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol. 35:11933–11938; 2014. [DOI] [PubMed] [Google Scholar]
- 13. Shishodia G.; Shukla S.; Srivastava Y.; Masaldan S.; Mehta S.; Bhambhani S.; Sharma S.; Mehrotra R.; Das B. C.; Bharti A. C. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Mol. Cancer 14:116; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bumrungthai S.; Ekalaksananan T.; Evans M. F.; Chopjitt P.; Tangsiriwatthana T.; Patarapadungkit N.; Kleebkaow P.; Luanratanakorn S.; Kongyingyoes B.; Worawichawong S.; Pientong C. Up-regulation of miR-21 is associated with cervicitis and human papillomavirus infection in cervical tissues. PLoS One 10:e0127109; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribeiro J.; Marinho-Dias J.; Monteiro P.; Loureiro J.; Baldaque I.; Medeiros R.; Sousa H. miR-34a and miR-125b expression in HPV infection and cervical cancer development. Biomed. Res. Int. 2015:304584; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He Y.; Lin J.; Ding Y.; Liu G.; Luo Y.; Huang M.; Xu C.; Kim T. K.; Etheridge A.; Lin M.; Kong D.; Wang K. A systematic study on dysregulated microRNAs in cervical cancer development. Int. J. Cancer 138:1312–1327. 2016. [DOI] [PubMed] [Google Scholar]
- 17. Jia H.; Zhang Z.; Zou D.; Wang B.; Yan Y.; Luo M.; Dong L.; Yin H.; Gong B.; Li Z.; Wang F.; Song W.; Liu C.; Ma Y.; Zhang J.; Zhao H.; Li J.; Yu J. MicroRNA-10a is down-regulated by DNA methylation and functions as a tumor suppressor in gastric cancer cells. PLoS One 9:e88057; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Li Z.; Lei H.; Luo M.; Wang Y.; Dong L.; Ma Y.; Liu C.; Song W.; Wang F.; Zhang J.; Shen J.; Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer 18:43–54; 2015. [DOI] [PubMed] [Google Scholar]
- 19. Huang L.; Lin J. X.; Yu Y. H.; Zhang M. Y.; Wang H. Y.; Zheng M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS One 7:e33762; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohuchida K.; Mizumoto K.; Lin C.; Yamaguchi H.; Ohtsuka T.; Sato N.; Toma H.; Nakamura M.; Nagai E.; Hashizume M.; Tanaka M. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Ann. Surg. Oncol. 19:2394–2402; 2012. [DOI] [PubMed] [Google Scholar]
- 21. Ma L.; Teruya-Feldstein J.; Weinberg R. A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449:682–688; 2007. [DOI] [PubMed] [Google Scholar]
- 22. Ma L. Role of miR-10b in breast cancer metastasis. Breast Cancer Res. 12:210; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasayama T.; Nishihara M.; Kondoh T.; Hosoda K.; Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int. J. Cancer 125:1407–1413; 2009. [DOI] [PubMed] [Google Scholar]
- 24. Kim K.; Lee H. C.; Park J. L.; Kim M.; Kim S.; Noh S. M.; Song K. S.; Kim J. C.; Kim Y. S. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics 6:740–751; 2011. [DOI] [PubMed] [Google Scholar]
- 25. Kosaka N.; Iguchi H.; Ochiya T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortez M. A.; Bueso-Ramos C.; Ferdin J.; Lopez-Berestein G.; Sood A. K.; Calin G. A. MicroRNAs in body fluids the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 8:467–477; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor D. D.; Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110:13–21; 2008. [DOI] [PubMed] [Google Scholar]
- 28. Garzon R.; Pichiorri F.; Palumbo T.; Iuliano R.; Cimmino A.; Aqeilan R.; Volinia S.; Bhatt D.; Alder H.; Marcucci G.; Calin G.A.; Liu C. G.; Bloomfield C. D.; Andreeff M.; Croce C. M. MicroRNA fingerprints during human megakaryocytopoiesis. Proc. Natl. Acad. Sci. USA 103:5078–5083; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hung Y. C.; Ueda M.; Terai Y.; Kumagai K.; Ueki K.; Kanda K.; Yamaguchi H.; Akise D.; Ueki M. Homeobox gene expression and mutation in cervical carcinoma cells. Cancer Sci. 94:437–441; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shim C.; Zhang W.; Rhee C. H.; Lee J. H. Profiling of differentially expressed genes in human primary cervical cancer by complementary DNA expression array. Clin. Cancer Res. 4:3045–3050; 1998. [PubMed] [Google Scholar]