Abstract
Gastric cancer is the fourth most common type of cancer and second leading cause of cancer-related death in the world. Since patients are often diagnosed at a late stage, very few effective therapies are left in the arsenal. FGF19, as a hormone, has been reported to promote tumor growth in various types of cancer; however, its function in gastric cancer remains unknown. In the current study, we showed that FGF19 is overexpressed in gastric cancer and is associated with depth of invasion, lymph node metastasis, and TNM stage. In addition, in vitro experiments demonstrated that FGF19 is able to enhance migration and invasion abilities of gastric cancer cells. Given its great potency in gastric cancer progression, FGF19 may be an effective target of treatment for advanced gastric cancer patients.
Key words: Gastric cancer, FGF19, Migration, Invasion
INTRODUCTION
Gastric cancer is an aggressive disease that continues to have a daunting impact on global health. Despite a decline in the global incidence in certain regions of the world, gastric cancer remains the fourth most common type of cancer and is the second leading cause of cancer-related death worldwide (1). The development of gastric cancer is a complex and multifactorial process involving a number of factors, such as Helicobacter pylori infection, high salt intake, smoking, and in a small percentage of patients, a familial genetic component (2). Gastric cancer is often diagnosed at an advanced stage. The cornerstone of therapy is surgical resection with adjuvant chemotherapy or chemoradiation in appropriate cases, but the 5-year survival of patients remains low, at 20–40% (3). Gastric cancer is a heterogeneous disease that demands continued attention and research with regard to prevention, early detection, and novel therapeutic options.
Fibroblast growth factor 19 (FGF19) is a protein that in humans is encoded by the FGF19 gene that belongs to the FGF family. It functions as a hormone, regulating bile acid synthesis, with effects on glucose and lipid metabolism (4–6). Recent studies have indicated significantly higher expression of FGF19 in tumor tissues compared with adjacent normal tissues, in several cancers, including human thyroid cancer (7), liver cancer (8), hepatocellular carcinoma (9), and prostate cancer (10). Functionally, it may be involved in the cancer progression. For example, Zhang et al. revealed that the increased expression of FGF19 might be involved in the malignant behaviors of thyroid cancer (7). Similarly, Feng et al. indicated that FGF19 silencing in prostate cancer cells decreased invasion and proliferation in vitro and tumor growth in vivo (10). In addition, the FGF19–FGFR4 signaling network may play an important role in the development and progression of cancers (11).
Abnormal FGF19 expression in tumor tissues suggests that FGF19 is significant in cancer pathogenesis and progression. However, the biological role of FGF19 in gastric cancer remains an enigma. To investigate the clinical significance of FGF19 and to examine whether it plays any roles in progression of gastric cancer, we performed immunohistochemistry to evaluate the expression of FGF19. Subsequently, the associations between FGF19 expression and clinicopathologic characteristics were statistically evaluated. After that, we examined the effects of FGF19 on tumor migration and invasion in vitro. Our findings suggest that plays an oncogenic role in gastric cancer, and therapeutic inactivation of FGF19 could be beneficial for the treatment of gastric cancer.
MATERIALS AND METHODS
Patients and Tissue Specimens
The current study was authorized by the Research Ethics Committee of Taian Central Hospital, China. All patients agreed to the procedure and signed consent forms. A total of 116 sets of gastric tumors and adjacent, nontumor tissues were resected for gastric cancer treatment at Taian Central Hospital between 2010 and 2014. The adjacent normal tissues were obtained over 5 cm from the primary cancer. The patients were 91 men and 25 women, with a mean age of 58.3 years (range: 28–81 years). None of the patients had received radiotherapy or chemotherapy before surgery. All cases of gastric cancer were histologically diagnosed according to the Japanese Classification of Gastric Carcinoma by at least two expert pathologists, working independently in a double-blinded fashion.
Immunohistochemistry
Paraffin-embedded tissue sections from gastric cancer specimens were given a heat pretreatment of 60°C for 1 h, then deparaffinized with xylene, rehydrated, and subjected to microwave antigen retrieval in citrate buffer (pH 6.0) for 30 min. After inhibition of endogenous peroxidase activity for 30 min with methanol containing 0.3% H2O2, the sections were incubated with the monoclonal anti-FGF19 antibody (1:500 dilution, SC-73984; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The experimental procedure was according to the manufacturer’s instructions of LSAB+ kit (Dako, Carpinteria, CA, USA).
Tissue sections were counterstained with Mayer’s hematoxylin. Slides were independently evaluated by two pathologists, blinded to any patient’s data. The intensity of staining was divided into four grades (intensity scores): no staining (0), weak staining (1), moderate staining (2), and strong staining (3). The percentage of positive cells was divided into five grades (percentage scores): <5% (0), 5–25% (1), 25–50% (2), 50–75% (3), and 75–100% (4). Immunohistochemistry stain score = positive cell score + staining intensity score. The overall score ≤3 was defined as negative and >3 as positive.
Cell Lines and Culture Conditions
The human gastric cancer cell lines MKN-28, MKN-45, SGC-7901, and AGS were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. BGC-823, NCI-N87 gastric cancer cell lines and the immortalized normal gastric mucosal epithelial cell line GES-1 were purchased from the American Type Culture Collection (ATCC). Cell lines were routinely maintained in RPMI-1640 medium containing 10% calf serum, 100 IU/ml streptomycin, and 100 IU/ml penicillin in a 5% CO2 atmosphere at 37°C.
Total RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s manual. RNA (1 μg) was reversely transcribed to cDNA using Reverse Transcription system (Promega, Madison, WI, USA). qRT-PCR was performed to quantify the mRNA levels of FGF19 with the SYBR Green PCR core reagent kit (Applied Biosystems, Foster City, CA, USA). GAPDH was used as the endogenous reference. The PCR primers designed for FGF19 were 5′-CGGAGGAAGACTGTGCTTTCG-3′ (forward) and 5′-CTCGGATCGGTACACATTGTAG-3′ (reverse); for GAPDH, 5′-GGACCTGACCTGCCGTCTAG-3′ (forward) and 5′-GTAGCCCAGGATGCCCTTGA-3′ (reverse) according to the human FGF19 and GAPDH cDNA sequences in GenBank. Relative expression of FGF19 in each cell line was analyzed using the comparative Ct method.
Western Blot Analysis
Protein extraction was done by RIPA buffer supplemented with protease inhibitors cocktail (Sigma-Aldrich, St. Louis, MO, USA), and protein quantification was performed by BCA Protein Assay kit (Pierce, Rockford, IL, USA). Protein (50 μg) was separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were blocked in TBS-T buffer containing 5% nonfat dry milk and hybridized with a primary antibody. The primary antibodies used were rabbit anti-FGF19 (1:100 dilution, SC-73984; Santa Cruz Biotechnology), rabbit anti-GAPDH (1:5,000 dilution, Ab9485; Abcam). Membranes were then washed three times in TBS-Tween solution for 15 min. Finally, membranes were incubated with HRP-conjugated secondary antibody. Protein bands were visualized using ECL reagent (Thermo) on a Tanon detection system.
RNA Interference of FGF19
Nontargeting negative-control small interfering RNA, human FGF19-specific siRNA, and lipofectamine were diluted separately in serum-free medium and then incubated at room temperature for 5 min. The diluted DNA and lipofectamine were then mixed and incubated at room temperature for 20 min. Aliquots of the transfection mixture were then added to the cells. Cells were verified and used for analysis 48 h after transfection.
Wound Healing Assay
Cells were seeded in six-well plates and cultured until they reached confluence. Wound healing assays were performed by scratching the cell monolayer with a sterile 20-μl pipette tip and then cultured with fresh DMEM medium containing 1% fetal calf serum. Wound closing was observed every 24 h.
Cell Invasion and Migration Assays
Cell migration and invasion were analyzed using a Transwell chamber assay (Corning). For migration, MKN-45 and NCI-N87 cells transfected with FGF19siRNA and nontargeting negative-control siRNA were added to the upper chamber with serum-free medium, and medium containing 10% fetal calf serum was added to the lower chamber. For the invasion assay, transfected cells were resuspended in 200 μl serum-free RPMI-1640 medium and placed into the upper chamber of the insert with Matrigel (BD Biosciences). After culture, cells remaining attached to the upper surface of the filters were carefully removed with cotton swabs. Invaded cells were stained with 1% crystal violet for 20 min at room temperature and examined by light microscopy.
Statistical Analysis
Data were expressed as mean ± SD and subjected to statistical analysis via SPSS Version 19.0 using the Student’s t-test. Correlations between FGF19 expression in gastric cancer tissues and clinicopathological parameters were analyzed by chi-square or Fisher’s exact tests. Differences were considered statistically significant in two-tailed tests with p < 0.05, and a value of p < 0.01 was considered highly significant.
RESULTS
Increased Expression of FGF19 Protein in Human Gastric Cancer Tissues
To explore the role of FGF19 in gastric cancer, we performed IHC to analyze the protein expression of FGF19 in 116 pairs of gastric cancer and adjacent nontumor tissues. Positive immunostaining of FGF19 protein was observed in 91 (78.4%) of 116 patients with gastric cancer, while it was seen faintly or with no staining in nontumor tissues. The FGF19 protein was localized in cytoplasm with or without membrane of malignant cells, as shown in Figure 1A. Based on our immunochemistry scoring system, we find the immunoreactive score of FGF19 protein in gastric cancer was much higher than nontumor tissues (p < 0.01) (Fig. 1B). Furthermore, statistical analysis showed that the presence of the FGF19 protein was significantly correlated with clinicopathological parameters. As shown in Table 1, the presence of FGF19 was associated with depth of invasion (p = 0.027), lymph node metastasis (p = 0.003), and TNM stage (p = 0.036), but not with other clinicopathological factors including age, sex, and tumor location, etc.
Figure 1.
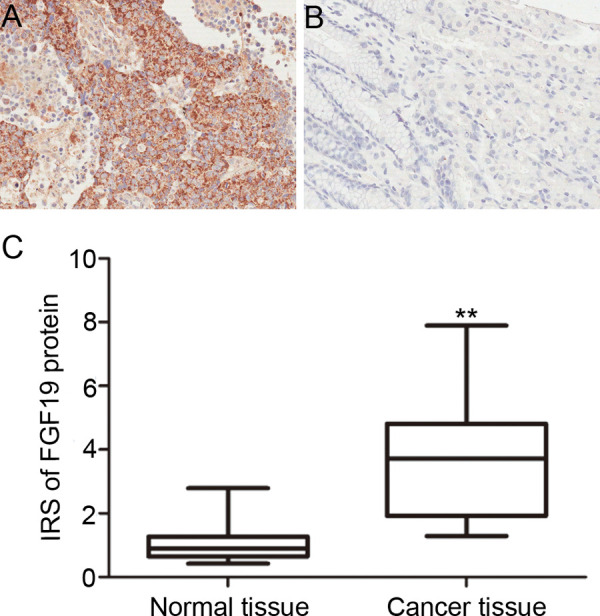
Immunostaining of FGF19 protein in human gastric tissues (200×). (A) Positive FGF19 expression in gastric cancer. (B) Negative FGF19 expression in nontumor gastric mucosa. (C) Expression level of FGF19 protein in gastric cancer tissues was significantly higher than in adjacent noncancerous tissues (p < 0.001).
Table 1.
Association Between FGF19 Expression and Clinicopathological Factors of Gastric Cancer Patients
| Variables | No. of Cases | FGF19 Immunostaining | p Value | |
|---|---|---|---|---|
| Positive (n = 91) | Negative (n = 25) | |||
| Gender | NS | |||
| Male | 91 | 71 | 20 | |
| Female | 25 | 20 | 5 | |
| Age (years) | NS | |||
| ≥65 | 27 | 21 | 6 | |
| <65 | 89 | 70 | 19 | |
| Tumor differentiation | NS | |||
| Well to moderate | 40 | 33 | 7 | |
| Poor | 76 | 58 | 18 | |
| Tumor location | NS | |||
| Gastric fundus | 6 | 4 | 2 | |
| Gastric corpus | 53 | 42 | 11 | |
| Pylorus | 57 | 45 | 12 | |
| Tumor size | NS | |||
| ≤5 m | 73 | 56 | 17 | |
| >5 m | 43 | 35 | 8 | |
| T stage | 0.027 | |||
| T1 + T2 | 20 | 12 | 8 | |
| T3 + T4 | 96 | 79 | 17 | |
| Lymph node metastasis | 0.003 | |||
| Negative | 33 | 20 | 13 | |
| Positive | 83 | 71 | 12 | |
| Distant metastasis | NS | |||
| Negative | 109 | 85 | 24 | |
| Positive | 7 | 6 | 1 | |
| TNM stage | 0.036 | |||
| I + II | 44 | 30 | 14 | |
| III + IV | 72 | 61 | 11 | |
NS, no significant difference between groups.
FGF19 Expression in Gastric Cancer Cell Lines
We investigated the expression of FGF19 in gastric cell lines and the noncancer cell line, GES-1, by qRT-PCR and Western blot analysis. The expression of FGF19 was higher both in mRNA level (Fig. 2A) and protein level (Fig. 2B) in six gastric cancer cell lines compared with immortalized gastric mucosa cell line GES-1. Among these six gastric cancer cell lines, MKN-45 and NCI-N87 cells expressed relatively more abundant FGF19.
Figure 2.
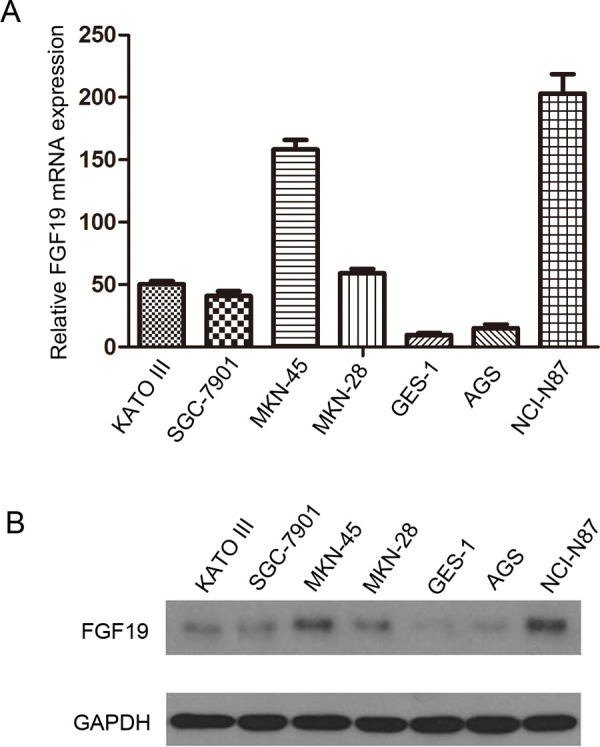
The expression of FGF19 in human gastric cell lines. (A) qRT-PCR analysis of FGF19 mRNA in six gastric cancer cell lines and GES-1. (B) Western blotting analysis of FGF19 protein in six gastric cancer cell lines and GES-1. The 24-kD band was detected by anti-FGF19 antibody (upper line), and the 42-kD band was detected by anti-GAPDH antibody (lower line) as loading control.
Expression of FGF19 Gene in Transfectants
To investigate the functions of FGF19 in gastric cancer cell lines, we performed targeted knockdown of FGF19 in the high-expressing cell lines MKN-45 and NCI-N87. The expressions of FGF19 gene in MKN-45/si-FGF19 and NCI-N87/si-FGF19 cells were detected using qRT-PCR (Fig. 3A) and Western blotting analysis (Fig. 3B). The results of qRT-PCR and Western blotting showed that the expression of FGF19 gene significantly decreased in MKN-45/si-FGF19 and NCI-N87/si-FGF19 cells when compared with the MKN-45/si-NC and NCI-N87/si-NC cells, respectively.
Figure 3.
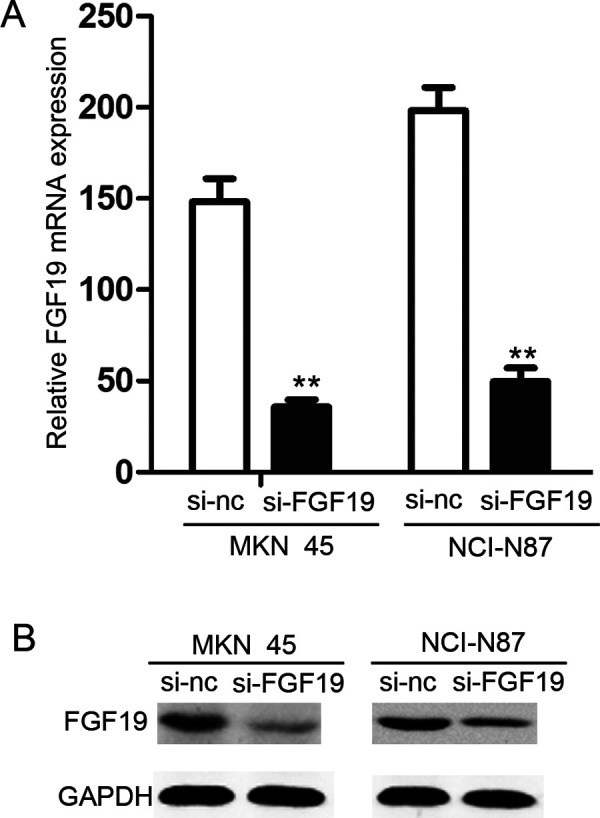
Downregulation of FGF19 in MKN-45 and NCI-N87 cells. (A) qRT-PCR analysis of FGF19 mRNA in MKN-45/si-FGF19, NCI-N87/si-FGF19, and their control groups. (B) Western blotting analysis of FGF19 protein in whole cell lysates using anti-FGF19, anti-GAPDH antibody.
Downregulation of FGF19 Inhibits Wound Healing Ability of Gastric Cancer Cells
A wound healing assay was used to examine migration ability of MKN-45 and NCI-N87. Cells were plated in a six-well plate after being transfected and scratched by 20-μl sterile pipette tips. Results are shown in Figure 4A. After 48-h incubation, the distance of the scratch wound in the MKN-45/si-FGF group was significantly larger compared with that of control groups. Similar results were observed in the NCI-N87 cell line (Fig. 4B).
Figure 4.
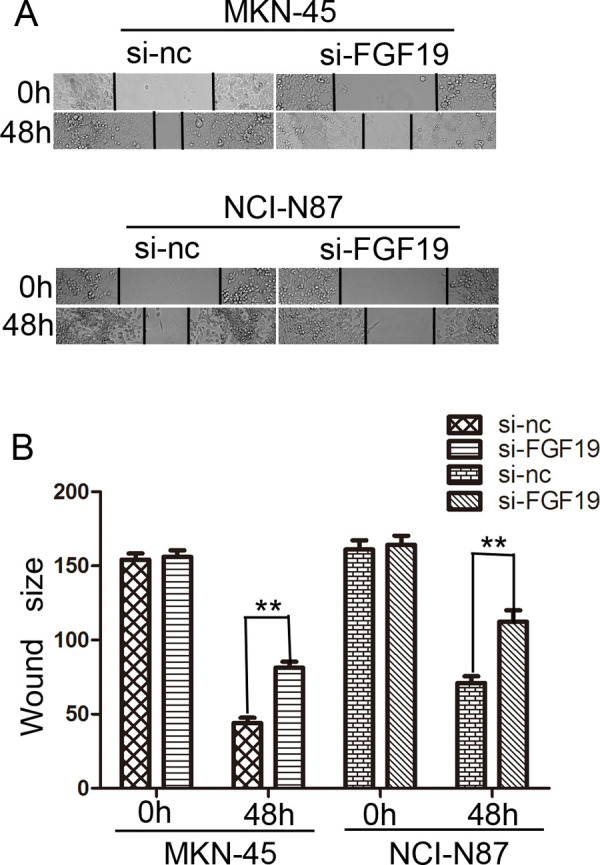
Effects of FGF19 on wound healing in gastric cancer cells (100×). (A) Wound healing assay with gastric cancer cells. Microscopic observations were recorded 0 and 48 h after scratching the cell surface. A representative image from every independent experiment is shown. (B) The size between wound edges of gastric cancer cells at 0 and 48 h. **p < 0.01.
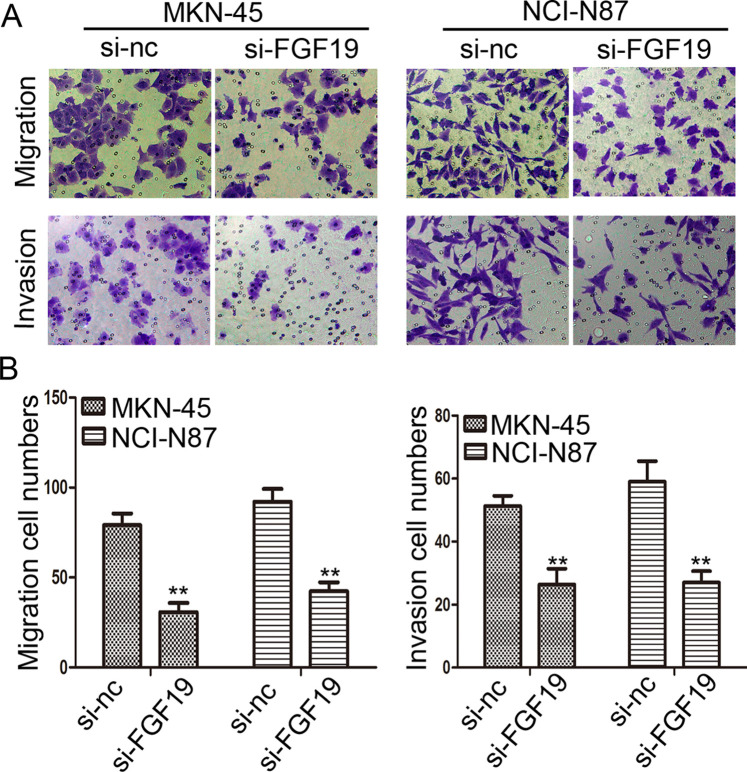
Downregulation of FGF19 Expression Suppresses Migration and Invasion Abilities of Gastric Cancer Cells
The above statistical analysis showed that FGF19 overexpression was significantly associated with depth of invasion, lymph node metastasis, and TNM stage, which prompted us to further confirm whether FGF19 plays any direct role in enhancing the migration and invasion abilities of gastric cancer cells. In the Transwell migration assay, the number of cells migrating through the chamber in MKN-45/si-FGF19 was 30.67 ± 5.13, which was lower than in the MKN-45/si-NC group (79.00 ± 6.56). The same trend was also observed in the invasion assay: MKN-45/si-FGF19 group: 26.33 ± 5.13, MKN-45/si-NC group: 51.33 ± 3.21 (p < 0.01) (Fig. 5A, B). Consistent results were also shown in NCI-N87 cell lines (p < 0.01) (Fig. 5A, B) (Migration assay: NCI-N87/si-FGF19 group: 42.33 ± 4.93, NCI-N87/si-NC group: 92.00 ± 7.21; Invasion assay: NCI-N87/si-FGF19 group: 27.00 ± 3.61, NCI-N87/si-NC group: 59.00 ± 6.56). These results suggest a functional role for FGF19 in gastric cancer metastasis.
Figure 5.
Knocking down FGF19 expression inhibits the migration and invasion of gastric cells in vitro. (A) Transwell assay. Photographs show cells that traveled through the micropore membrane with or without Matrigel. (B) Histograms showed the numbers of migration cells and invasion cells. **p < 0.01.
DISCUSSION
In the current study, we, for the first time, showed that FGF19 is overexpressed in gastric cancer and is closely associated with depth of invasion, lymph node metastasis, and TNM stage. In addition, FGF19 is able to enhance migration and invasion abilities of gastric cancer cells, which is consistent with studies of other cancer types (10).
Although there have been studies focusing on the mechanism of its role in tumor growth (9,12), a perspicuous picture of FGF19’s mechanism, especially that of migration and invasion, remains largely elusory. Some studies showed that FGF19 and CCND1 are coamplified in liver cancer (13); since cyclin has a predominant role in cell cycle and tumor growth, it may not be the putative signaling pathway in cancer migration and invasion. In addition, the downstream effector of FGF19 has been established to be FGFR4 in liver cancer (14), and its signal transduction may need assists from a-Klotho (KL) and/or b-Klotho (KLB), two related single-pass transmembrane proteins (15,16); however, it is unclear whether FGFR4 and its coreceptors are still involved in gastric cancer in regard to migration and invasion abilities. More importantly, the activated downstream signaling pathway responsible for the increasing migration and invasion in gastric cancer is still a moot point at present, which needs further investigation.
As an oncogene, the reason for its dysregulation in gastric cancer remains unknown. Besides amplicons and overexpression discovered in liver cancer (8), other upstream transcription factors or micro-RNA may be attributed to FGF19’s upregulation. Being a secretive protein, FGF19 may exert its function through paracrine, autocrine, and endocrine (17,18) and become an influential factor in the tumor microenvironment. Such a growth factor may be activated by upstream regulators and, eventually, initiate a signaling cascade that promotes tumor development and progression.
The ability of migration and invasion is of great importance to cancer cells, especially for gastric cancer. Since most patients diagnosed are already at an advanced stage and eventually die from remote metastasis, an effective target of migration and invasion will shed great light on gastric cancer treatment. In this study, we demonstrated that FGF19 is upregulated in gastric cancer and correlated to aggressive properties. Its proinvasive and promigration ability was proved by in vitro experiments. We will continue to investigate the detailed mechanisms of this promising target and, hopefully, bring real benefit to gastric cancer patients in the near future.
REFERENCES
- 1. Jemal A.; Bray F.; Center M. M.; Ferlay J.; Ward E.; Forman D. Global cancer statistics. CA Cancer J. Clin. 61:69–90; 2011. [DOI] [PubMed] [Google Scholar]
- 2. Correa P. Gastric cancer: Overview. Gastroenterol. Clin. North Am. 42:211–217; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu L.; Duan Y. T.; Li J. F.; Su L. P.; Yan M.; Zhu Z. G.; Liu B. Y.; Yang Q. M. Biglycan enhances gastric cancer invasion by activating Fak signaling pathway. Oncotarget 5:1885–1896; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu X.; Ge H.; Lemon B.; Vonderfecht S.; Weiszmann J.; Hecht R.; Gupte J.; Hager T.; Wang Z.; Lindberg R.; Li Y. Fgf19-induced hepatocyte proliferation is mediated through Fgfr4 activation. J. Biol. Chem. 285:5165–5170; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schaap F. G. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 15:386–391; 2012. [DOI] [PubMed] [Google Scholar]
- 6. Jones S. Mini-review: Endocrine actions of fibroblast growth factor 19. Mol. Pharm. 5:42–48; 2008. [DOI] [PubMed] [Google Scholar]
- 7. Zhang X.; Wang Z.; Tian L.; Xie J.; Zou G.; Jiang F. Increased expression of Fgf19 contributes to tumor progression and cell motility of human thyroid cancer. Otolaryngol. Head Neck Surg. 154:52–58; 2016. [DOI] [PubMed] [Google Scholar]
- 8. Sawey E. T.; Chanrion M.; Cai C.; Wu G.; Zhang J.; Zender L.; Zhao A.; Busuttil R. W.; Yee H.; Stein L.; French D. M.; Finn R. S.; Lowe S. W.; Powers S. Identification of a therapeutic strategy targeting amplified Fgf19 in liver cancer by oncogenomic screening. Cancer Cell 19:347–358; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desnoyers L. R.; Pai R.; Ferrando R. E.; Hotzel K.; Le T.; Ross J.; Carano R.; D’Souza A.; Qing J.; Mohtashemi I.; Ashkenazi A.; French D. M. Targeting Fgf19 inhibits tumor growth in colon cancer xenograft and Fgf19 transgenic hepatocellular carcinoma models. Oncogene 27:85–97; 2008. [DOI] [PubMed] [Google Scholar]
- 10. Feng S.; Dakhova O.; Creighton C. J.; Ittmann M. Endocrine fibroblast growth factor Fgf19 promotes prostate cancer progression. Cancer Res. 73:2551–2562; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurosu H.; Choi M.; Ogawa Y.; Dickson A. S.; Goetz R.; Eliseenkova A. V.; Mohammadi M.; Rosenblatt K. P.; Kliewer S. A.; Kuro-o M. Tissue-specific expression of betaklotho and fibroblast growth factor (Fgf) receptor isoforms determines metabolic activity of Fgf19 and Fgf21. J. Biol. Chem. 282:26687–26695; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicholes K.; Guillet S.; Tomlinson E.; Hillan K.; Wright B.; Frantz G. D.; Pham T. A.; Dillard-Telm L.; Tsai S. P.; Stephan J. P.; Stinson J.; Stewart T.; French D. M. A mouse model of hepatocellular carcinoma: Ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 160:2295–2307; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibcus J. H.; Kok K.; Menkema L.; Hermsen M. A.; Mastik M.; Kluin P. M.; van der Wal J. E.; Schuuring E. High-resolution mapping identifies a commonly amplified 11q13.3 region containing multiple genes flanked by segmental duplications. Hum. Genet. 121:187–201; 2007. [DOI] [PubMed] [Google Scholar]
- 14. Wu X.; Li Y. Therapeutic utilities of fibroblast growth factor 19. Expert Opin. Ther. Targets 15:1307–1316; 2011. [DOI] [PubMed] [Google Scholar]
- 15. Kurosu H.; Kuro O. M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol. Cell Endocrinol. 299:72–78; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Pai R.; Dunlap D.; Qing J.; Mohtashemi I.; Hotzel K.; French D. M. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating beta-catenin signaling. Cancer Res. 68:5086–5095; 2008. [DOI] [PubMed] [Google Scholar]
- 17. Kwabi-Addo B.; Ozen M.; Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr. Relat. Cancer 11:709–724; 2004. [DOI] [PubMed] [Google Scholar]
- 18. Turner N.; Grose R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 10:116–129; 2010. [DOI] [PubMed] [Google Scholar]