Figure 6.
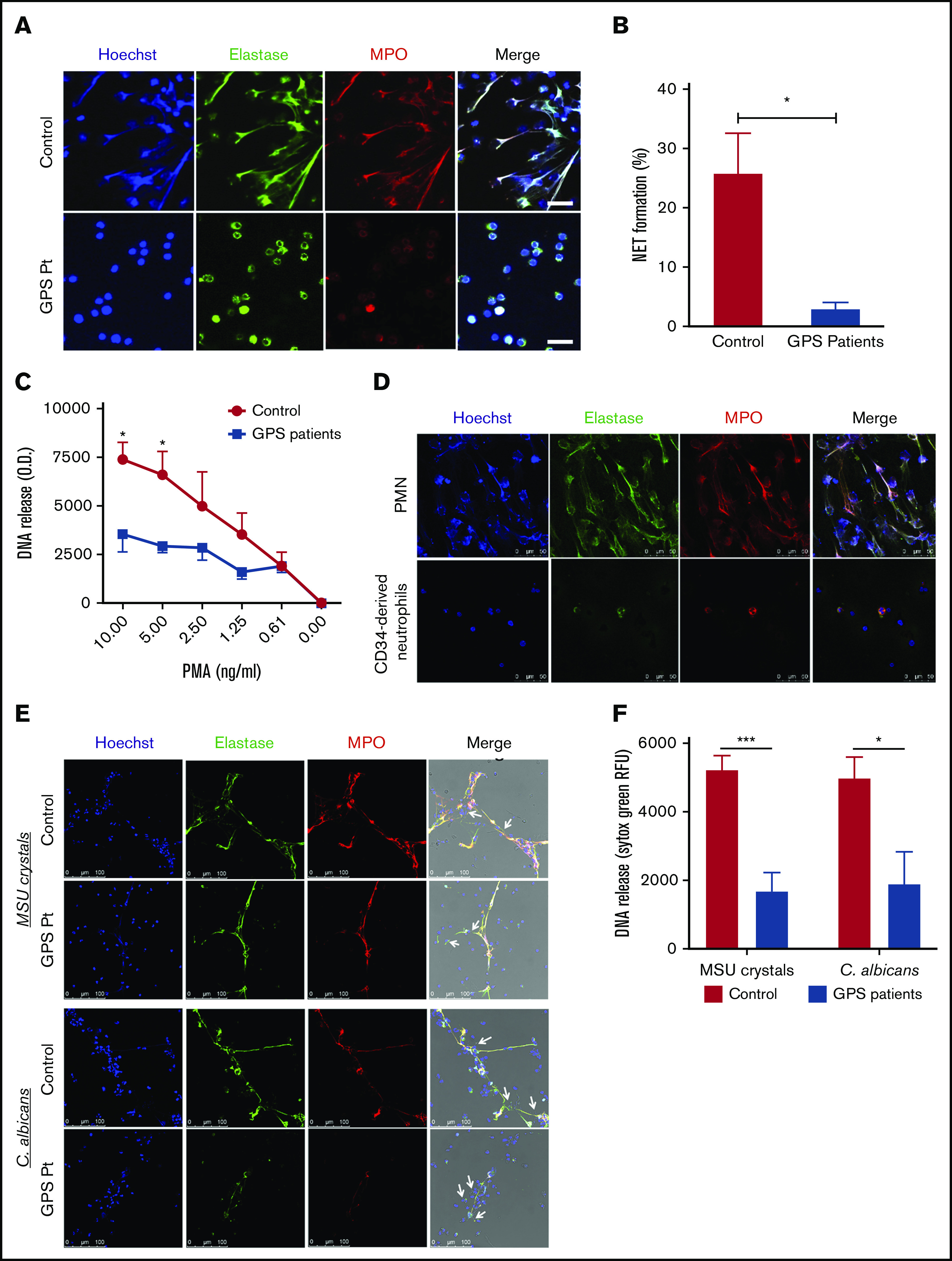
Impaired NETosis by GPS neutrophils and MPB CD34+HSC-derived neutrophils. (A) Neutrophils derived from blood from controls (upper panel) and patients with GPS (lower panel) were stimulated with PMA (100 ng/mL) for 3 hours, and NET formation was visualized by staining for DNA (blue), elastase (green), and MPO (red). Scale bars, 20 µm. (B) NET formation was determined by counting the number of NETs per field of 100 cells in control and GPS neutrophils, stimulated with PMA (100 ng/mL) for 3 hours, in ≥5 fields. (C) DNA release was measured in the supernatant from control and GPS neutrophils stimulated with increasing concentrations of PMA (n = 3-5). (D) Control neutrophils from blood (PMN, upper panel) or MPB CD34+ HSC-derived neutrophils (lower panel) were stimulated with PMA (100 ng/mL) for 3 hours, and NET formation was visualized by staining for DNA (Hoechst, blue), elastase (green), and MPO (red). Shown are representative images of n = 3. Scale bars, 50 µm. (E) Neutrophils derived from blood from controls and patients with GPS were stimulated with MSU crystals (200 μg/mL, upper panel) or opsonized C albicans (lower panel) for 4 hours. NET formation was visualized by staining for DNA (blue), elastase (green), and MPO (red). White arrows in the merge images indicate the MSU crystals (upper panel) or the C albicans hyphae (lower panel). Scale bars, 100 µm. Shown are representative images of n = 3-9. Pt, patient. (F) DNA release was measured in the supernatant from control (n = 7-9) and GPS (n = 3-4) neutrophils stimulated with MSU crystals (200 μg/mL) or opsonized C albicans for 4 hours. RFU, relative fluorescence units. Results are means ± SEM. An unpaired, 2-tailed t test was used. *P < .05, ***P < .001 compared with control neutrophils.
