Figure 1.
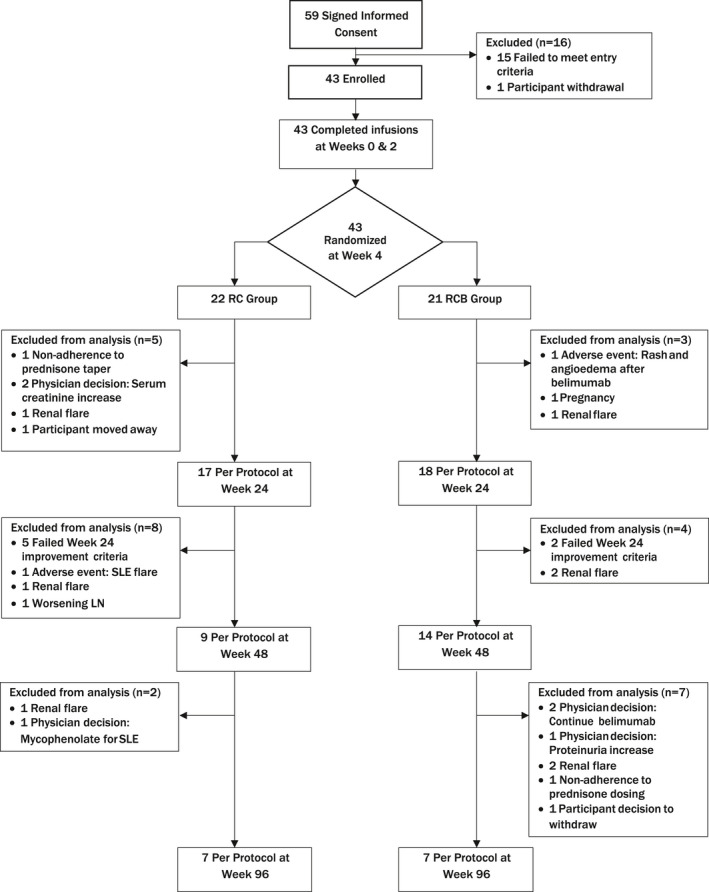
Flow diagram according to the Consolidated Standards of Reporting Trials (CONSORT) statement, showing the distribution of patients with recurrent or refractory lupus nephritis (LN) at each stage of the study from the time of informed consent to week 96. Reasons for exclusion of patients at each stage are provided. Samples from the per protocol population were evaluated at weeks 24, 48, and 96. RC = treatment with rituximab and cyclophosphamide but no belimumab infusions; RCB = treatment with rituximab, cyclophosphamide, and glucocorticoids followed by weekly belimumab infusions until week 48; SLE = systemic lupus erythematosus.
