Figure 1.
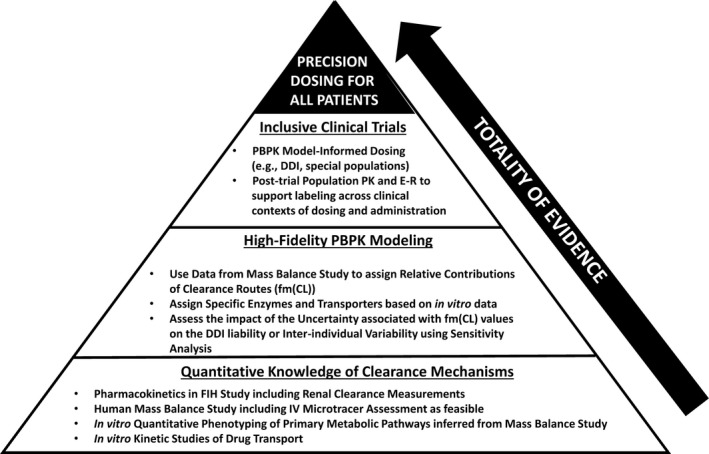
Roadmap for precision dosing based on the totality of evidence. A pyramid view of the roadmap consists of the following key elements: the base of the pyramid relates to quantitative assessment of clearance mechanisms based on MB (mass balance) data and relevant in vitro studies; the middle of the pyramid pertains to the development of a high‐fidelity PBPK model informed by the MB and in vitro data; in the upper level of the pyramid, predictions from a high‐fidelity PBPK model can inform optimal dosing across clinical trials. DDI, drug–drug interactions; E–R, exposure–response; FIH, first‐in‐human; fm(Cl), contributions of routes to overall clearance; IV, intravenous; PBPK, physiologically‐based pharmacokinetic; PK, pharmacokinetics.
