Abstract
Aim
To evaluate the efficacy and safety of adjunct dapagliflozin therapy in patients with type 1 diabetes (T1D).
Materials and Methods
DEPICT‐1 and ‐2 were randomized, double‐blind, parallel‐group, 24‐week studies, with 28‐week extension periods. Adults with T1D and HbA1c 7.5%‐10.5% were randomized (1:1:1) to receive dapagliflozin 5 mg, 10 mg or placebo. The short‐ and long‐term efficacy and safety of dapagliflozin were examined in an exploratory pooled analysis of both studies.
Results
Efficacy analyses included 530, 529 and 532 and safety analysis included 548, 566 and 532 patients in the dapagliflozin 5 mg, 10 mg and placebo groups, respectively. Baseline characteristics were similar between treatment groups. At week 24, reductions were seen with dapagliflozin 5 and 10 mg compared with placebo in HbA1c (−0.40%, −0.43% vs. 0.00%) and body weight (−2.45, −2.91 vs. 0.11 kg). HbA1c and body weight reductions versus placebo were also seen after 52 weeks of treatment. There was no imbalance in occurrence of severe hypoglycaemic events between groups. The proportion of patients experiencing definite diabetic ketoacidosis (DKA) was higher with dapagliflozin 5 mg (4.0%) and 10 mg (3.5%) compared with placebo (1.1%) over 52 weeks; most events were of mild or moderate severity, and all resolved with treatment.
Conclusions
Over 52 weeks, dapagliflozin provided glycaemic and weight benefits, with no increased frequency of severe hypoglycaemia compared with placebo. More DKA events were reported with dapagliflozin than placebo, highlighting the importance of appropriate patient selection, education and risk‐mitigation strategies.
Keywords: dapagliflozin, DEPICT, DKA, insulin adjunct, long‐term data, severe hypoglycaemia, SGLT‐2 inhibitor, T1D
1. INTRODUCTION
Patients with type 1 diabetes (T1D) require life‐long insulin therapy to maintain glycaemic control. 1 However, the majority of the affected population fail to achieve glycaemic targets, with only ~30% of adults with T1D achieving HbA1c of less than 7.0%. 2 There is a need for treatments acting through insulin‐independent pathways that can be used as add‐on therapy in patients with T1D to improve glycaemic control without causing hypoglycaemia and weight gain.
DEPICT‐1 and ‐2 were phase III, randomized, double‐blind, parallel‐group, 24‐week studies, with 28‐week extension periods, investigating the efficacy and safety of dapagliflozin used as an adjunct to adjustable insulin therapy in adult patients with T1D and uncontrolled hyperglycaemia. 3 , 4 , 5 Based on the results of the DEPICT studies, the sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitor dapagliflozin (5 mg dose) has been approved by the European Medicines Agency for use as an adjunct to adjustable insulin in patients with T1D and body mass index (BMI) of 27 kg/m2 or higher. 6 In Japan, dapagliflozin 5 and 10 mg doses have been approved for use as an adjunct to insulin therapy for patients with T1D, regardless of BMI. 7 , 8
Results from the DEPICT studies showed that adjunct dapagliflozin therapy resulted in improved HbA1c and reduced body weight, without an increase in the risk of hypoglycaemia; however, there is a lack of reported findings on whether the benefit/risk balance of dapagliflozin treatment differs among patients with different characteristics, for example, high or very high HbA1c at baseline, or the method of insulin administration.
The aim of this analysis was to report the safety and efficacy of adjunct dapagliflozin therapy in a pooled population from the DEPICT‐1 and ‐2 studies. In addition, we aimed to explore whether the efficacy and safety of dapagliflozin treatment differs between subgroups of patients according to baseline HbA1c (<75 mmol/mol [<9.0%] or ≥75 mmol/mol [≥9.0%]) or method of insulin administration (continuous subcutaneous insulin infusion [CSII] or multiple daily injections [MDI]).
2. METHODS
2.1. Study design
This was a pooled analysis of the DEPICT‐1 (NCT02268214) and DEPICT‐2 (NCT02460978) studies. Study designs for DEPICT‐1 and DEPICT‐2 have been described previously. 3 , 4 , 5 Both studies included an 8‐week lead‐in period to optimize diabetes management; a 24‐week, double‐blind treatment period (short‐term period); a 28‐week subject‐ and site‐blinded extension (long‐term period); and a 4‐week follow‐up period.
2.2. Participants
In brief, this pooled analysis included participants aged 18‐75 years with inadequately controlled T1D (HbA1c 7.7%‐11.0% [61‐97 mmol/mol] at screening/enrolment; 7.5%‐10.5% [58‐91 mmol/mol] at randomization), BMI 18.5 kg/m2 or higher, and C‐peptide less than 0.7 ng/mL (<0.23 nmol/L). Participants were required to have been taking insulin for 12 months or longer prior to screening, without changing the method of administration for 3 months or longer, and with a total insulin dose of 0.3 U/kg/day or more, and to be receiving CSII, or three or more injections of insulin/day if following a MDI regimen. Patients were excluded if they previously used any SGLT‐2 inhibitor, had type 2 diabetes (T2D), maturity onset diabetes of the young, pancreatic disorders resulting in decreased β‐cell capacity, diabetes insipidus, diabetic ketoacidosis (DKA) requiring medical intervention within 1 month before screening, or had been hospitalized for hyperglycaemia or hypoglycaemia within 1 month. A detailed list of inclusion and exclusion criteria for patients participating in the DEPICT trials has been published previously. 3 , 4 , 5
2.3. Procedures
Eligible patients were randomized (1:1:1) to receive dapagliflozin 5 mg, dapagliflozin 10 mg or placebo in addition to insulin, with randomization stratified by use of continuous glucose monitoring (CGM), method of insulin administration and baseline HbA1c. Throughout the study, insulin dose could be adjusted according to self‐monitored blood‐glucose readings, local guidance and individual circumstances. Basal and bolus insulin doses were recommended to be reduced by up to 20% on day 1, before attempting to titrate back to baseline levels. Change in mode of insulin administration was not allowed except temporarily, for up to 2 weeks, in certain circumstances, such as when a patient using an insulin pump needed a replacement pump and the temporary use of MDI was allowed. Up to week 24, each insulin dose was recorded every day during select 2‐week collection periods. At other times, and from week 24 and 52, patients recorded the midpoint for basal and bolus insulin doses each week.
2.4. Outcomes
2.4.1. Efficacy
Efficacy outcomes for this pooled analysis were mean change from baseline in HbA1c (primary efficacy outcome for the DEPICT‐1 and DEPICT‐2 studies at week 24), mean % changes from baseline in total daily insulin dose (TDID) and body weight, mean change from baseline in seated systolic blood pressure (SBP) in patients with hypertension at baseline (SBP ≥ 140 mmHg and/or seated diastolic blood pressure ≥ 90 mmHg), and proportion of patients achieving an HbA1c reduction of 0.5% or higher without severe hypoglycaemia. Efficacy findings were reported for the short‐ and long‐term treatment periods. Data were also reported for the 30‐day post‐treatment follow‐up visit, if available.
Subgroup analyses were conducted to assess the difference versus placebo in HbA1c, TDID and body weight in subgroups of patients with baseline HbA1c less than 75 mmol/mol (<9.0%) or 75 mmol/mol or higher (≥9.0%), or according to the method of insulin administration (CSII or MDI). Placebo‐adjusted differences for HbA1c and body weight for the subgroup analyses were reported for week 24 and 52. Difference versus placebo for TDID for the subgroups was only reported for week 24, as between week 24 and 52, patients only recorded the midpoint for basal and bolus insulin dose each week and comparative analysis was not conducted for this period.
2.4.2. Safety
Safety assessments, including adverse events (AEs) and serious AEs (SAEs), were monitored throughout the long‐term study period, including up to the week 56 follow‐up visit, and are reported for the pooled population as well as for the subgroups. AEs of special interest, including hypoglycaemia, DKA, genital infections, urinary tract infections, fractures and cardiovascular AEs, were also recorded.
Details of assessing hypoglycaemia and DKA have been described previously. 3 , 4 , 5 Hypoglycaemic events were defined according to the American Diabetes Association (ADA) classification criteria. 9 , 10 Severe hypoglycaemia was defined as an event resulting in unconsciousness caused by hypoglycaemia or an event requiring the assistance of another person to administer carbohydrate, glucagon, or to take other corrective actions to promote neurological recovery. 9
Patients were advised on identifying potential DKA events, and were provided with blood ketone meters with instructions for their use to record ketone levels. Potential DKA events were identified based on investigator review of home ketone meter readings, review of symptoms and diagnoses, and from queries according to predefined terms in the Standardized Medical Dictionary for Regulatory Activities. An independent DKA Adjudication Committee adjudicated all reported DKA events. Criteria for definite DKA included acidosis diagnosed by low blood pH (<7.3) and/or decreased serum bicarbonate levels (≤18 mEq/L). Symptoms/signs listed in the ADA consensus statement on the diagnosis of DKA were also assessed. 11 There were no adjudication criteria for possible and improbable DKA events.
2.5. Statistical analysis
Data from patients in both DEPICT studies were pooled for this analysis. All efficacy analyses, including analyses for the subgroups, were performed using the full analysis set (defined as all correctly randomized patients who received ≥1 dose of the study medication during the 24‐week period and who had baseline and any postbaseline assessments). In DEPICT‐1, 55 patients were incorrectly and non‐randomly allocated by the interactive voice‐response system to the dapagliflozin treatment arms because of a randomization system error. These patients are included in the safety analysis set based on the treatment received (18 patients received ≥1 dose of dapagliflozin 5 mg and 37 subjects received ≥1 dose of dapagliflozin 10 mg), but they were removed from the full analysis set for the efficacy analyses.
Change and % change from baseline for the overall population and difference versus placebo for the subgroup analyses were assessed using a longitudinal repeated measures analysis. Independent variables for the overall population analyses were baseline value, treatment, study, study week, randomization stratification factor, the interaction between study week and treatment, and the interaction between study week and baseline value. Independent variables for the subgroup analyses were baseline value, treatment, study, study week, randomization stratification factor, the interaction between study week and treatment, the interaction between study week and baseline value, subgroup, the interaction between treatment and subgroup, the interaction between study week and subgroup, and the interaction between treatment, study week and subgroup.
Logistic regression was used to calculate the proportion of patients achieving an HbA1c reduction of 0.5% or higher without severe hypoglycaemia with adjustment for the randomization stratification factor and baseline HbA1c values. For missing data, the last observation carried forward (LOCF) method was used. Odds ratios (OR), based on logistic regression, for other endpoints and their associated 95% confidence intervals (CI), were determined for dapagliflozin (both doses) versus placebo. No P‐values were calculated for treatment group comparisons, as all efficacy analyses for the pooled dataset were exploratory.
No formal analysis was conducted to compare TDID between the groups beyond week 24 because different methods were used to record the data.
Safety outcomes were summarized descriptively and assessed using the safety analysis set that included all patients receiving one or more doses of study medication during the short‐term, double‐blind treatment period. Safety analyses were performed over 52 weeks, plus the 30‐day follow‐up period.
3. RESULTS
3.1. Patients
Of the 1646 patients randomized at the initiation of the short‐term periods of the DEPICT‐1 (n = 833) and DEPICT‐2 (n = 813) studies, 1464 patients (88.9%) entered the long‐term extensions (DEPICT‐1: n = 747; DEPICT‐2: n = 717). The full analysis set for this pooled population included 530, 529 and 532 patients in the dapagliflozin 5 mg, 10 mg and placebo groups, respectively, and the safety analysis set included 548, 566 and 532 patients, respectively. Overall, 85.0%, 85.0% and 81.6% of patients in the dapagliflozin 5 mg, 10 mg and placebo groups, respectively, completed the 52‐week treatment period. The main reasons for study discontinuation were occurrence of AEs (1.9%) and patients requesting to discontinue (1.0%). Baseline characteristics were similar between treatment groups (Table S1).
3.2. Efficacy outcomes
Reductions in HbA1c from baseline were observed at week 24 and 52 with both dapagliflozin doses, with minimal changes in the placebo group (Figure 1A). Adjusted mean (SE) change in HbA1c from baseline was −0.40 (0.03) % for dapagliflozin 5 mg, −0.43 (0.03) % for dapagliflozin 10 mg and 0.00 (0.04) % for placebo at week 24, and −0.20 (0.04) % for dapagliflozin 5 mg, −0.23 (0.04) % for dapagliflozin 10 mg and 0.07 (0.04) % for placebo at week 52. There were, in total, 154 patients (47 in the dapagliflozin 5 mg group, 47 in the dapagliflozin 10 mg group and 60 in the placebo group) that were missing HbA1c data at week 24 and there were 286 patients (86 in the dapagliflozin 5 mg group, 92 in the dapagliflozin 10 mg group and 108 in the placebo group) that were missing HbA1c data at week 52; LOCF was utilized in these patients.
FIGURE 1.
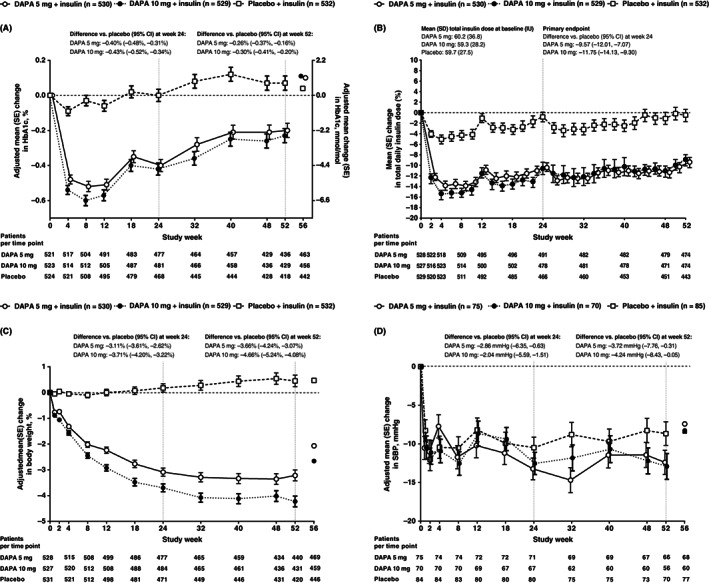
A, adjusted mean (SE) change from baseline to week 52 in HbA1c, B, adjusted mean (SE) % change from baseline to week 52 in total daily insulin dose, C, adjusted mean (SE) % change from baseline to week 52 in body weight and D, adjusted mean (SE) change from baseline to week 52 in SBP (for patients with hypertension at baseline) (full analysis set). For HbA1c, body weight and SBP, adjusted mean change or % change is shown up to week 52. For insulin dose, up to week 24 insulin was recorded daily at set times, but from week 24 to 52 patients recorded the midpoint for basal and bolus insulin dose for each week. Therefore, B represents the mean % change over time (descriptive only); for HbA1c, body weight and SBP, values at week 56 (post‐treatment follow‐up visit) are mean change; for HbA1c and SBP, mixed model: change from baseline = baseline treatment study week stratum week*treatment week*baseline; for insulin dose and body weight, mixed model: log(post) – log (baseline) = log(baseline) treatment study week stratum week*treatment week*log(baseline); stratum includes one term for each combination of all stratification factors. DAPA, dapagliflozin; SBP, systolic blood pressure
TDID decreased from baseline by week 2 with dapagliflozin 5 and 10 mg, and remained lower than placebo in both dapagliflozin groups over the 52 weeks of treatment (Figure 1B). Mean (SD) TDID at baseline for patients included in this analysis was 60.2 (36.8) IU with dapagliflozin 5 mg, 59.3 (28.2) IU with dapagliflozin 10 mg and 59.7 (27.5) IU with placebo (Figure 1B). Adjusted mean (SE) % change in TDID from baseline was −8.30 (0.97) % for dapagliflozin 5 mg, −10.51 (0.96) % for dapagliflozin 10 mg and 1.40 (1.09) % for placebo at week 24. Basal and bolus daily insulin doses were similarly reduced at week 24 compared with placebo in patients who received either dose of dapagliflozin. Adjusted mean (SE) % change from baseline to week 24 in basal insulin was −11.50 (0.97) % for dapagliflozin 5 mg, −15.33 (0.94) % for dapagliflozin 10 mg and 0.18 (1.12) % for placebo (difference [95% CI] vs. placebo: dapagliflozin 5 mg, −11.65% [−14.15, −9.08]; dapagliflozin 10 mg, −15.48% [−17.87, −13.02]). Adjusted mean (SE) % change from baseline to week 24 in bolus insulin was −12.83 (1.45) % with dapagliflozin 5 mg, −13.11 (1.46) % with dapagliflozin 10 mg and −3.46 (1.64) % with placebo (difference [95% CI] vs. placebo: dapagliflozin 5 mg, −9.70% [−13.54, −5.69]; dapagliflozin 10 mg, −9.99% [−13.83, −5.98]).
Both dapagliflozin doses had greater reductions in body weight at week 24 compared with placebo; this was also seen after 52 weeks of treatment, with further reductions in body weight seen with dapagliflozin compared with placebo (Figures 1C, S1). Adjusted mean (SE) % change from baseline in body weight was −3.10 (0.18) % (−2.45 [0.14] kg) for dapagliflozin 5 mg, −3.70 (0.18) % (−2.91 [0.14] kg) for dapagliflozin 10 mg and 0.02 (0.19) % (0.11 [0.14] kg) for placebo at week 24 and −3.22 (0.21) % (−2.57 [0.18] kg) for dapagliflozin 5 mg, −4.23 (0.21) % (−3.34 [0.18] kg) for dapagliflozin 10 mg and 0.45 (0.22) % (0.44 [0.18] kg) for placebo at week 52.
At baseline, 230 patients had hypertension (defined as SBP ≥ 140 mmHg and/or seated diastolic blood pressure ≥ 90 mmHg; dapagliflozin 5 mg: n = 75; dapagliflozin 10 mg: n = 70; placebo: n = 85), with mean (SD) baseline SBP values in these patients of 145.52 (9.66) mmHg in the dapagliflozin 5 mg group, 145.81 (9.60) mmHg in the dapagliflozin 10 mg group and 144.11 (10.85) mmHg in the placebo group (Figure 1D). Adjusted mean (SE) change from baseline in SBP was −13.30 (1.40) mmHg for dapagliflozin 5 mg, −12.47 (1.43) mmHg for dapagliflozin 10 mg and − 10.44 (1.36) mmHg for placebo at week 24 and −12.39 (1.58) mmHg for dapagliflozin 5 mg, −12.91 (1.67) mmHg for dapagliflozin 10 mg and −8.67 (1.54) mmHg for placebo at week 52.
The proportion of patients achieving an HbA1c reduction of 0.5% or higher without severe hypoglycaemia was greater with dapagliflozin 5 and 10 mg than with placebo after 24 and 52 weeks of treatment (Figure S2).
Overall, similar trends were seen in the subgroup analyses in patients with baseline HbA1c of less than 75 mmol/mol (<9.0%) or 75 mmol/mol or higher (≥9.0%) and those using MDI or CSII. At week 24, placebo‐adjusted differences from baseline for HbA1c, body weight and TDID were comparable between the overall pooled population and the subgroups of patients with different baseline HbA1c (Figure 2), as well as MDI and CSII users (Figure 3). Differences versus placebo in HbA1c and body weight were also observed after 52 weeks of treatment in the subgroups.
FIGURE 2.
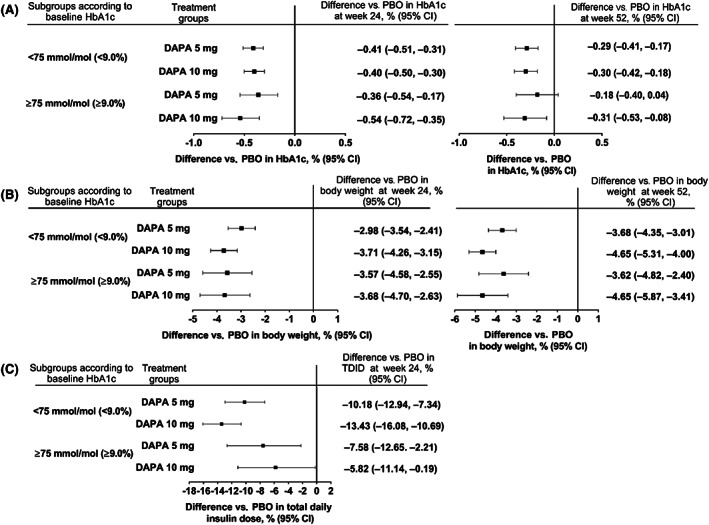
Difference versus placebo with dapagliflozin 5 mg and 10 mg for A, change from baseline in HbA1c at week 24 and 52, B, % change from baseline in body weight at week 24 and 52, C, % change from baseline in total daily insulin dose at week 24 in subgroups of patients with baseline HbA1c < 75 mmol/mol (<9.0%) or ≥75 mmol/mol (≥9.0%). The mixed model included terms for baseline, treatment, study, week, stratum, week*treatment, week*baseline, subgroup, treatment*subgroup, week*subgroup and treatment*week*subgroup. Stratum includes one term for each combination of the three stratification factors on baseline HbA1c, use of personal continuous glucose monitoring system and methods of insulin administration. CI, confidence interval; DAPA, dapagliflozin; PBO, placebo; TDID, total daily insulin dose
FIGURE 3.
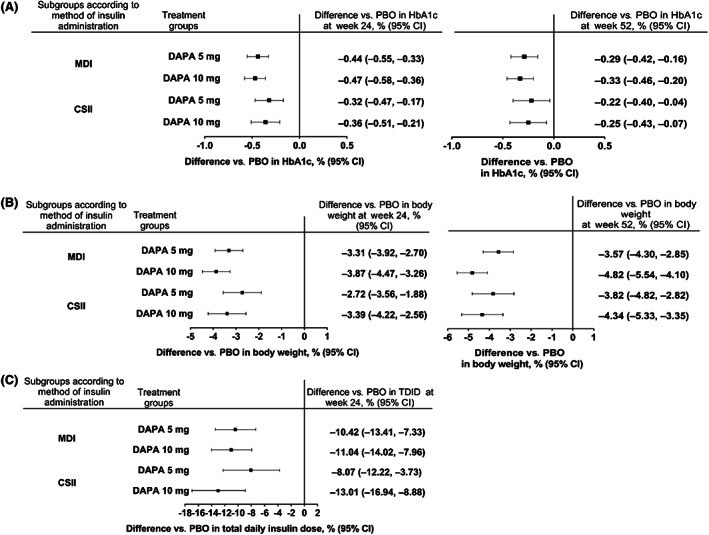
Difference versus placebo with dapagliflozin 5 and 10 mg for A, change from baseline in HbA1c at week 24 and 52, B, % change from baseline in body weight at week 24 and 52, C, % change from baseline in total daily insulin dose at week 24 in subgroups of patients receiving insulin either via continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI). The mixed model included terms for baseline, treatment, study, week, stratum, week*treatment, week*baseline, subgroup, treatment*subgroup, week*subgroup and treatment*week*subgroup. Stratum includes one term for each combination of the three stratification factors on baseline HbA1c, use of personal continuous glucose monitoring system and methods of insulin administration. CI, confidence interval; DAPA, dapagliflozin; PBO, placebo; TDID, total daily insulin dose
3.3. Safety outcomes
Over the study period (52 weeks plus 30‐day follow‐up), one or more AE was reported for 79.9%, 77.9% and 74.1% of patients in the dapagliflozin 5 mg, 10 mg and placebo groups, respectively, and one or more SAE was reported in 12.6%, 10.4% and 8.6% of patients, respectively (Table 1). More genital infections were reported for dapagliflozin 5 and 10 mg than with placebo over 24 and 52 weeks of treatment. The proportion of patients reporting AEs and SAEs over the entire study period was comparable between the overall pooled population and the subgroups of patients with baseline HbA1c of less than 75 mmol/mol (<9.0%) or 75 mmol/mol or higher (≥9.0%) and those receiving insulin via CSII or MDI (Tables S2 and S3).
TABLE 1.
Safety outcomes a for the DEPICT pooled population
| Week 24 (short‐term period) | Week 52 (short‐term + long‐term period) | |||||
|---|---|---|---|---|---|---|
| DAPA 5 mg (N = 548) | DAPA 10 mg (N = 566) | Placebo (N = 532) | DAPA 5 mg (N = 548) | DAPA 10 mg (N = 566) | Placebo (N = 532) | |
| AEs | ||||||
| ≥1 AE | 384 (70.1) | 388 (68.6) | 332 (62.4) | 438 (79.9) | 441 (77.9) | 394 (74.1) |
| ≥1 AE related to study drug | 157 (28.6) | 153 (27.0) | 63 (11.8) | 181 (33.0) | 180 (31.8) | 88 (16.5) |
| AE leading to study discontinuation | 23 (4.2) | 20 (3.5) | 20 (3.8) | 35 (6.4) | 30 (5.3) | 27 (5.1) |
| AE of special interest b | ||||||
| Adjudicated CV event | 2 (0.4) | 5 (0.9) | 2 (0.4) | 2 (0.4) | 5 (0.9) | 4 (0.8) |
| Events of renal function | 6 (1.1) | 2 (0.4) | 0 | 7 (1.3) | 3 (0.5) | 4 (0.8) |
| Fracture | 8 (1.5) | 6 (1.1) | 5 (0.9) | 12 (2.2) | 11 (1.9) | 12 (2.3) |
| Urinary tract infection | 37 (6.8) | 21 (3.7) | 25 (4.7) | 57 (10.4) | 30 (5.3) | 39 (7.3) |
| Male c | 2 (0.8) | 2 (0.7) | 3 (1.2) | 4 (1.7) | 4 (1.5) | 4 (1.6) |
| Female d | 35 (11.3) | 19 (6.5) | 22 (7.8) | 53 (17.0) | 26 (8.9) | 35 (12.5) |
| Genital infection | 61 (11.1) | 54 (9.5) | 12 (2.3) | 73 (13.3) | 68 (12.0) | 18 (3.4) |
| Male c | 12 (5.1) | 12 (4.4) | 0 | 13 (5.5) | 17 (6.2) | 0 |
| Female d | 49 (15.8) | 42 (14.3) | 12 (4.3) | 60 (19.3) | 51 (17.4) | 18 (6.4) |
| SAEs | ||||||
| ≥1 SAE | 37 (6.8) | 31 (5.5) | 20 (3.8) | 69 (12.6) | 59 (10.4) | 46 (8.6) |
| ≥1 SAE related to study drug | 18 (3.3) | 12 (2.1) | 3 (0.6) | 23 (4.2) | 20 (3.5) | 6 (1.1) |
| SAE leading to study discontinuation | 15 (2.7) | 7 (1.2) | 6 (1.1) | 22 (4.0) | 13 (2.3) | 9 (1.7) |
| Hypoglycaemia | ||||||
| ≥1 SAE of hypoglycaemia | 6 (1.1) | 2 (0.4) | 2 (0.4) | 8 (1.5) | 5 (0.9) | 5 (0.9) |
| Hypoglycaemia leading to study discontinuation | 3 (0.5) | 0 | 1 (0.2) | 3 (0.5) | 1 (0.2) | 2 (0.4) |
| Ketone‐related events e | ||||||
| ≥1 ketone‐related SAE | 14 (2.6) | 11 (1.9) | 2 (0.4) | 28 (5.1) | 20 (3.5) | 4 (0.8) |
| Ketone related SAE leading to study discontinuation | 9 (1.6) | 7 (1.2) | 0 | 14 (2.6) | 11 (1.9) | 0 |
| Death f | 0 | 0 | 1 (0.2) | 1 (0.2) | 0 | 1 (0.2) |
Abbreviations: AE, adverse event; CV, cardiovascular; DAPA, dapagliflozin; DKA, diabetic ketoacidosis; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
24‐week data include non‐SAEs seen from day 1 of treatment up to and including 4 days after the last dose in the short‐term period; 52‐week data also include events up to and including 30 days after the last dose in the short‐ +long‐term period.
Based on a list of prespecified list of MedDRA preferred terms.
Denominator is the number of male patients in the DAPA 5 mg (n = 237), DAPA 10 mg (n = 273) and placebo (n = 251) groups.
Denominator is the number of female patients in the DAPA 5 mg (n = 311), DAPA 10 mg (n = 293) and placebo (n = 281) groups.
Preferred terms for ketone‐related events were acetonaemia, acidosis, ketoacidosis, ketosis, metabolic acidosis, uraemic acidosis, DKA, diabetic metabolic decompensation, blood ketone body, blood ketone body increased, urine ketone body present, blood ketone body present, urine ketone body, diabetic ketoacidotic hyperglycaemic coma, ketonuria, diabetic ketosis, euglycaemic DKA.
One death in the DAPA 5 mg group was attributed to an SAE that was not related to the study treatment.
The total number of hypoglycaemic and severe hypoglycaemic events were similar in all treatment groups over 24 and 52 weeks (Table S4). Hypoglycaemia events, including severe hypoglycaemia, were also comparable between the overall populations and baseline HbA1c subgroups or MDI versus CSII users (Tables S2 and S3).
Over 52 weeks, more patients in the dapagliflozin groups than in the placebo group had events adjudicated as definite DKA (Table 2). Of the definite DKA events, 36.7% (18/49) were adjudicated as mild, 40.8% (20/49) as moderate and 22.4% (11/49) as severe. There was also a higher number of euglycaemic DKA events in the dapagliflozin group compared with the placebo group. The identified primary causes of DKA over the 52‐week period were missed insulin dose (34.8% [8/23] with dapagliflozin 5 mg, 25.0% [5/20] with dapagliflozin 10 mg and 16.7% [1/6] with placebo), insulin pump failure (17.4% [4/23] with dapagliflozin 5 mg, 25.0% [5/20] with dapagliflozin 10 mg and 33.3% [2/6] with placebo) and severe illness (4.3% [1/23] with dapagliflozin 5 mg, 5.0% [1/20] with dapagliflozin 5 mg and 0% with placebo). The cause of definite DKA was ‘other’ or ‘not identified’ in 43.4% (10/23) of cases with dapagliflozin 5 mg, 45% (9/20) with dapagliflozin 10 mg and 50% (3/6) with placebo. All DKA events were resolved with treatment.
TABLE 2.
Summary of DKA events a for the DEPICT pooled population
| Week 24 (short‐term period) | Week 52 (short‐term + long‐term period) | |||||
|---|---|---|---|---|---|---|
| DAPA 5 mg (N = 548) | DAPA 10 mg (N = 566) | Placebo (N = 532) | DAPA 5 mg (N = 548) | DAPA 10 mg (N = 566) | Placebo (N = 532) | |
| Patients with adjudicated definite DKA, n (%) | 11 (2.0) | 11 (1.9) | 3 (0.6) | 22 (4.0) | 20 (3.5) | 6 (1.1) |
| Events sent for adjudication, n | 54 | 41 | 28 | 81 | 76 | 40 |
| Number of events of definite DKA, n | 11 | 11 | 3 | 23 | 20 | 6 |
| Incidence rate, per 100 patient‐years | 4.55 | 4.36 | 1.29 | 4.62 | 3.85 | 1.27 |
| Severity of event as adjudicated, n | ||||||
| Mild | 5 | 4 | 1 | 9 | 6 | 3 |
| Moderate | 4 | 4 | 1 | 8 | 10 | 2 |
| Severe | 2 | 3 | 1 | 6 | 4 | 1 |
| Number of events of euglycaemic DKA b , n | 2 | 3 | 0 | 6 | 7 | 1 |
| Primary cause for definite DKA events, n | ||||||
| Insulin pump failure | 3 | 3 | 1 | 4 | 5 | 2 |
| Missed insulin dose | 3 | 4 | 1 | 8 | 5 | 1 |
| Severe illness | 0 | 0 | 0 | 1 | 1 | 0 |
| Not identified | 5 | 0 | 0 | 7 | 3 | 1 |
| Other | 0 | 4 | 1 | 3 | 6 | 2 |
| Mean total insulin dose reduction compared to baseline for week before definite DKA events, % | −13.94 | −23.29 | −7.79 | −3.57 | −21.05 | −10.14 |
| Mean total insulin dose reduction compared with baseline at end of treatment period in patients with definite DKA events, % | −13.80 | −22.39 | 30.76 | −12.08 | −20.43 | 5.07 |
| Events adjudicated as not DKA | ||||||
| Patients with possible DKA, n (%) | 11 (2.0) | 11 (1.9) | 3 (0.6) | 16 (2.9) | 13 (2.3) | 4 (0.8) |
| Number of events of possible DKA, n | 14 | 12 | 5 | 22 | 14 | 6 |
| Patients with improbable DKA, n (%) | 16 (2.9) | 12 (2.1) | 10 (1.9) | 19 (3.5) | 21 (3.7) | 12 (2.3) |
| Number of events of improbable DKA, n | 29 | 18 | 20 | 36 | 42 | 28 |
Abbreviations: DAPA, dapagliflozin; DKA, diabetic ketoacidosis.
24‐week data include non‐SAEs seen from day 1 of treatment up to and including 4 days after the last dose in the short‐term period; 52‐week data also include events up to and including 30 days after the last dose in the short‐ +long‐term period.
Euglycaemic DKA was defined as having a measured glucose below 250 mg/dL closest to the time of the highest beta‐hydroxybutyrate value.
The incidence of events adjudicated as definite DKA throughout the study period was comparable between baseline HbA1c subgroups for patients treated with dapagliflozin (Table S2). There were more events in patients treated with dapagliflozin than in those treated with placebo. Overall, there was a greater proportion of patients experiencing DKA events in the dapagliflozin groups and the placebo group when administering insulin via CSII compared with MDI (Table S3).
4. DISCUSSION
The DEPICT‐1 and DEPICT‐2 studies had near‐identical designs and showed consistent results, which allowed a pooled analysis. The analysis of this large pooled dataset investigated the use of adjunct SGLT‐2 therapy for the management of T1D. In this geographically diverse population of people with T1D, dapagliflozin provided glycaemic and weight benefits, along with reductions in insulin dose over 52 weeks. Here, we also provide evidence that the safety and efficacy of adjunct dapagliflozin therapy was consistent across baseline HbA1c subgroups and by the method of insulin administration.
Results of this pooled analysis showed a clinically relevant decrease in HbA1c with adjunct dapagliflozin therapy at week 24, which was also seen, although to a lesser extent, at week 52. In addition, the decrease in HbA1c was observed without an increase in the risk of hypoglycaemia or weight gain. Indeed, body weight had begun to decrease with dapagliflozin treatment by week 2 and remained lower than in the placebo group throughout the treatment period. Furthermore, reduction in HbA1c was seen despite reductions in TDID. With intensive insulin therapy being a major cause of weight gain in T1D, 12 the reduction in TDID without compromising blood glucose control is a desirable outcome in T1D management.
Reduction in body weight observed in the DEPICT studies may be caused by caloric loss from SGLT‐2–mediated glycosuria, as is the case in T2D. 13 The extent of any additional contribution of insulin dose reduction to weight loss is not clear. Importantly, weight loss has been associated with reduced cardiovascular co‐morbidity in patients with T1D, 14 therefore, the initiation of a treatment that has a potential to reduce body weight may also contribute to a reduction in the risk of cardiovascular disease.
The subgroup analyses according to the baseline HbA1c and method of insulin administration showed comparable efficacy results with the overall DEPICT population. In studies in patients with T2D, the glucose‐lowering effect of SGLT‐2 inhibitors was greater in subjects with high HbA1c levels compared with those with low HbA1c levels, 15 , 16 which was not observed in the patients with T1D investigated in the DEPICT trials. One possible reason for this could be that the patients with T1D in the DEPICT studies had insulin administered via MDI or CSII, and the dose could be freely adjusted throughout the day as needed. Compared with patients with T1D, patients with T2D receiving insulin therapy usually receive more stable doses of insulin, and in many cases do not use bolus insulin. This possibility to freely adjust insulin throughout the day in the DEPICT studies may have masked the full glycaemic potential of dapagliflozin in this population.
Improved glycaemic control has been associated with reduced microvascular and macrovascular complications. 17 , 18 , 19 Dapagliflozin has been shown to reduce hypertension, arterial stiffness and the risk of diabetic retinopathy in patients with T2D, all of which are common complications of T1D related to uncontrolled hyperglycaemia. 20 , 21 , 22 , 23 While there was a trend towards greater reductions in SBP seen in this analysis with dapagliflozin compared with placebo in patients with hypertension at baseline, no significant differences in SBP were observed across the study groups. The immediate reductions in SBP observed here may be a result of regression to the mean, as hypertension was defined as SBP of 140 mmHg or higher and/or seated diastolic blood pressure of 90 mmHg or higher at baseline, rather than a history of diagnosis of hypertension.
The safety profile of dapagliflozin in this pooled analysis of patients with T1D was generally consistent with that seen with dapagliflozin in patients with T2D. 24 , 25 There was, however, a 3‐fold increase in DKA reported with dapagliflozin compared with placebo. The DKA incidence was within the range reported for the general T1D population 26 and has also been seen in studies with other SGLT‐2 inhibitors. 27 , 28 The underlying causes of the increased DKA risk with SGLT‐2 treatment compared with placebo are not well known, 29 but there is some evidence that this might be associated with a failure to recognize early metabolic decompensation. 30 It has been shown that during insulin suspension, patients being treated with SGLT‐2 inhibitors have a much more gradual rate of increase in plasma glucose levels and reach a plateau, which may result in delayed recognition of metabolic decompensation. 30 Indeed, euglycaemic DKA was more common in those receiving dapagliflozin treatments. This suggests that clinicians and patients should not depend solely on the elevation in plasma glucose levels for the detection of DKA, but should also include the monitoring of blood or urine ketones and on whether there are any other symptoms, such as nausea, vomiting or dehydration. 31
The subgroup analyses of the DEPICT studies showed that there were more DKA events observed in those using CSII in both of the dapagliflozin groups and the placebo group compared with those using MDI. This was also found in a meta‐analysis of randomized controlled trials, where DKA was generally more common in those having CSII as the mode of insulin administration, regardless of the use of SGLT‐2 inhibitors. 32 Hence, the risk ratio is not higher in users of CSII for dapagliflozin versus placebo, but may only reflect DKA being more common in patients who use CSII versus MDI.
It should also be noted that at the time the studies were conducted, less was known regarding the risk of DKA, its management and appropriate patient education, than has since then become available. Learnings from these studies include the need for patient and physician education about possible risk factors for DKA, risk‐reduction strategies and management as well as reporting of DKA events, which should all be implemented before initiating adjunct SGLT‐2 inhibitor therapy in patients with T1D. 33 Selection of appropriate patients for adjunct SGLT‐2 inhibitor therapy should be based on patient lifestyle (e.g. treatment adherence, diet, alcohol consumption) and regular monitoring of ketones. It is important that patients taking these agents are educated to respond appropriately to elevated ketone levels to minimize the risk of DKA. 34 , 35 , 36 Clinicians and patients should also be aware that DKA may present in those receiving adjunct SGLT‐2 inhibitor therapy without overtly elevated glucose levels (euglycaemic DKA), which may delay diagnosis and treatment if not promptly recognized. 34
The DEPICT studies, and this pooled analysis, have some limitations. During both studies, insulin was recorded daily at set times up to week 24, but from week 24 to 52, patients recorded the midpoint for basal and bolus insulin dose for each week, therefore, comparing changes in the insulin dose over these periods was not possible. Furthermore, CGM devices were worn only prior to the week 24 visit, but not the week 52 visit; therefore, long‐term CGM outcomes were not available. The absence of a protocol‐mandated insulin titration algorithm, while more closely reflecting real‐world clinical practice and management, may have masked the full glycaemic potential of dapagliflozin. On the other hand, the LOCF method, used for imputing missing data, may have led to an overestimation of the effect size. Another limitation could be the absence of information on diet, including carbohydrate intake, during these studies. Finally, the monitoring of DKA and hypoglycaemia in real‐world situations may differ from that conducted in a randomized controlled trial setting, which includes carefully selected patients.
In conclusion, this pooled analysis of a large, geographically diverse population of patients with T1D showed that dapagliflozin was well tolerated and led to clinically relevant improvements in glycaemic control and weight reduction, without an increased risk of severe hypoglycaemia. Compared with placebo, the risk of DKA was higher in patients treated with dapagliflozin; however, this risk may be mitigated by appropriate patient selection, education and other risk‐mitigation strategies when treating adults with T1D with adjunct dapagliflozin therapy.
CONFLICT OF INTEREST
MP is a stock shareholder at NG Solutions Ltd., DreaMed‐Diabetes Ltd. and Nutriteen Professionals Ltd. He has received grants/research support from Medtronic, Novo Nordisk, Roche, Eli Lilly, Merck, Sanofi, Pfizer, Bristol‐Myers Squibb, OPKO, Dexcom, Insulet and Lexicon, and honoraria, consultation fees and speaker bureau from Sanofi, Medtronic, Novo Nordisk, Eli Lilly, Pfizer, RSP Systems, Qulab Medical, AstraZeneca, Lilly and Insulet. CM has served as a consultant or advisory board member for ActoBio Therapeutics, AstraZeneca, Boehringher Ingelheim, Eli Lily and Company, Merck Sharp and Dohme Ltd., Novo Nordisk, Roche and Sanofi, and has also received honorarium from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Novo Nordisk and Sanofi. ML has received research grants from AstraZeneca, DexCom, Novo Nordisk and Pfizer, and has been a consultant for or received honoraria from AstraZeneca, DexCom, Eli Lilly, Medtronic, MSD, Novo Nordisk and Rubin Medical. EA has participated in advisory panels for Abbott Japan, AstraZeneca, Novo Nordisk Pharma and Ono Pharmaceutical, has received grants from Astellas Pharma, Daiichi Sankyo Company, Eli Lilly Japan, Kowa Company, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Sanofi, Sumitomo Dainippon Pharma and Taisho Pharmaceutical Takeda Pharmaceutical, and personal fees from Arkray, Astellas Pharma, AstraZeneca, Daiichi Sankyo Company, Eli Lilly Japan, Fujifilm Pharmaceutical, Kissei Pharmaceutical, Kowa Company, Kyowa Kikaku, WebMD, Mitsubishi Tanabe Pharma, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Sumitomo Dainippon Pharma, Taisho Pharmaceutical and Takeda Pharmaceutical. PDB has served as a consultant or advisory board member for AstraZeneca, Boehringher Ingelheim, Eli Lily and Company, and Novo Nordisk, and has also received honorarium from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk, Abbott Diagnostic, Mundipharna and Sanofi. RB has received research support, consulted, or has been on the scientific advisory board for Abbott Diabetes Care, Astra Zeneca, DexCom, Hygieia, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, Senseonics and United Healthcare. RB's employer, the non‐profit HealthPartners Institute, contracts for his services and RB receives no personal income from this. SRH serves on advisory boards and provides consultancy for AstraZeneca, Sanofi‐Aventis, Boeringher‐Ingelheim, Eli Lilly, Novo‐Nordisk, Springer Medical, Zealand Pharma and UN‐EEG, for which his institution receives renumeration. He also serves on speaker panels for Novo‐Nordisk, for which he receives renumeration. LH is an employee and shareholder of Bristol‐Myers Squibb. JX is an employee and shareholder of AstraZeneca. PD serves on advisory boards for Abbvie, AstraZeneca, Boehringer Ingelheim, Merck Intarcia, Novo Nordisk and Sanofi. He has received research grants from Abbvie, AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. NA, NI and FT are employees of AstraZeneca. MFS was an employee of AstraZeneca during the current analyses and preparation of this manuscript and is currently employed at Bayer Pharmaceuticals.
AUTHOR CONTRIBUTIONS
All authors contributed to the study concept or design, analysis or interpretation of the study data. All authors contributed to the drafting of the manuscript, critical revision for intellectual content and approved the final version for submission. MP is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14248.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGEMENTS
The authors thank the participating adults with T1D, their families and all investigators involved in the DEPICT‐1 and ‐2 studies. This pooled analysis has been presented as an abstract and a poster at American Diabetes Association's 79th Scientific Sessions, 7–11 June 2019, San Francisco, California. Writing support was provided by Evelina Matekonyte, inScience Communications, Springer Healthcare Ltd, UK, and was funded by AstraZeneca. The DEPICT studies were sponsored by AstraZeneca and Bristol‐Myers Squibb. This pooled post hoc analysis was funded by AstraZeneca.
Phillip M, Mathieu C, Lind M, et al. Long‐term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: pooled 52‐week outcomes from the DEPICT‐1 and ‐2 studies. Diabetes Obes Metab. 2021;23:549–560. 10.1111/dom.14248
Funding information The DEPICT studies were sponsored by AstraZeneca and Bristol‐Myers Squibb. This pooled post hoc analysis was funded by AstraZeneca.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure..
REFERENCES
- 1. NICE guidelines . Type 1 diabetes in adults: diagnosis and management. 2016. https://bnf.nice.org.uk/treatment-summary/type-1-diabetes.html.
- 2. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971‐978. [DOI] [PubMed] [Google Scholar]
- 3. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24 week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):864‐876. [DOI] [PubMed] [Google Scholar]
- 4. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care. 2018;41(12):2552‐2559. [DOI] [PubMed] [Google Scholar]
- 5. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients With inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care. 2018;41(9):1938‐1946. [DOI] [PubMed] [Google Scholar]
- 6. EMC . Forxiga 5 mg film‐coated tablets: European Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/2865/smpc. Accessed August 28, 2019.
- 7. Araki E, Watada H, Uchigata Y, et al. Efficacy and safety of dapagliflozin in Japanese patients with inadequately controlled type 1 diabetes (DEPICT‐5): 52‐week results from a randomized, open‐label, phase III clinical trial. Diabetes Obes Metab. 2020;22(4):540‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Japanese Ministry of Health Labour and Welfare . Forxiga 5 and 10 mg product label. 2019. https://www.info.pmda.go.jp/go/pack/3969019F1027_2_10/?view=frame&style=XML&lang=ja. Accessed December 20, 2019.
- 9. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Workgroup on Hypoglycemia ADA . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association workgroup on hypoglycemia. Diabetes Care. 2005;28(5):1245‐1249. [DOI] [PubMed] [Google Scholar]
- 11. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mottalib A, Kasetty M, Mar JY, Elseaidy T, Ashrafzadeh S, Hamdy O. Weight management in patients with type 1 diabetes and obesity. Curr Diab Rep. 2017;17(10):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020‐1031. [DOI] [PubMed] [Google Scholar]
- 14. de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843‐2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yagi S, Aihara KI, Kondo T, et al. Predictors for the treatment effect of sodium glucose co‐transporter 2 inhibitors in patients with type 2 diabetes mellitus. Adv Ther. 2018;35(1):124‐134. [DOI] [PubMed] [Google Scholar]
- 16. Iemitsu K, Iizuka T, Takihata M, et al. Factors influencing changes in hemoglobin A1c and body weight during treatment of type 2 diabetes With ipragliflozin: interim analysis of the ASSIGN‐K study. J Clin Med Res. 2016;8(5):373‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long‐term mortality. JAMA. 2015;313(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972‐1982. [DOI] [PubMed] [Google Scholar]
- 19. Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ott C, Jumar A, Striepe K, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Boer IH, Kestenbaum B, Rue TC, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168(17):1867‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordin D, Rönnback M, Forsblom C, Heikkilä O, Saraheimo M, Groop PH. Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia. 2007;50(9):1808‐1814. [DOI] [PubMed] [Google Scholar]
- 23. Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(6):381‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32(9):1656‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilding JP, Woo V, Soler NG, et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405‐415. [DOI] [PubMed] [Google Scholar]
- 26. Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258‐2265. [DOI] [PubMed] [Google Scholar]
- 28. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560‐2569. [DOI] [PubMed] [Google Scholar]
- 29. Fattah H, Vallon V. The potential role of SGLT2 inhibitors in the treatment of type 1 diabetes mellitus. Drugs. 2018;78(7):717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel NS, Van Name MA, Cengiz E, et al. Altered patterns of early metabolic decompensation in type 1 diabetes during treatment with a SGLT2 inhibitor: an insulin pump suspension study. Diabetes Technol Ther. 2017;19(11):618‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium‐glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pala L, Dicembrini I, Mannucci E. Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta‐analysis of randomized clinical trials. Acta Diabetol. 2019;56(9):973‐980. [DOI] [PubMed] [Google Scholar]
- 33. Boeder S, Edelman SV. Sodium‐glucose co‐transporter inhibitors as adjunctive treatment to insulin in type 1 diabetes: a review of randomized controlled trials. Diabetes Obes Metab. 2019;21(Suppl 2):62‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium‐glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20(9):571‐575. [DOI] [PubMed] [Google Scholar]
- 36. Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium‐glucose co‐transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab. 2019;21(10):2192‐2202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure..


