Figure 3.
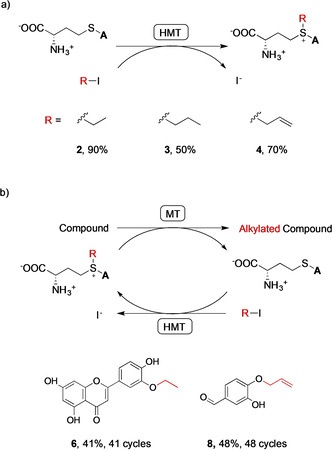
a) Preparative‐scale (15 mg) synthesis of SAE (2), SAP (3), and SAA (4) catalyzed by the V140T AtHMT. The “A” represents the adenosyl moiety. Conversions shown are from Table S4. b) Production (10–20 mg scale) of alkylated products using cyclic MT‐HMT cascades, employing 100 μm SAH and 80 mm alkyl iodide. The IeOMT variant T133M‐Y326L and the V140T AtHMT catalyzed the ethylation of luteolin and produced 4′‐O‐ethylluteolin (6) with 41 % conversion. Human COMT and V140T AtHMT catalyzed the allylation of 3,4‐dihydroxybenzaldehyde and produced 4‐allyloxy‐3‐hydroxybenzaldehyde (8) with 48 % conversion. Insignificant conversion took place if the HMT and MT were not added (Figures S9 and S11). Conversions are from Table S5 and numbers of SAH regeneration cycles were calculated as [product]/[SAH]t=0.
