FIGURE 8.
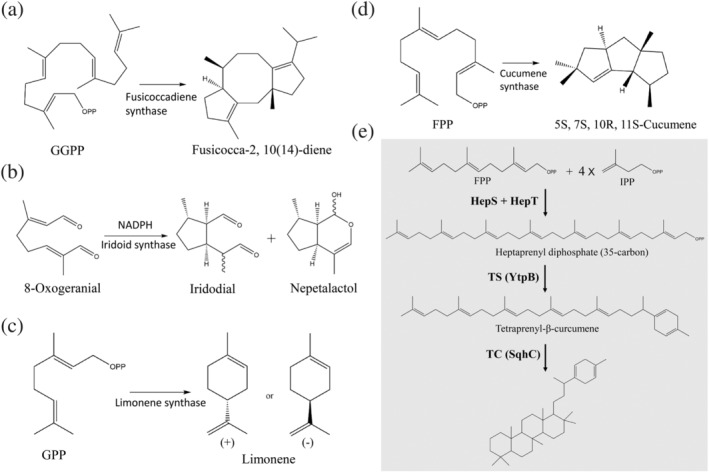
Certain critical cyclization reactions catalyzed by terpene cyclases, as shown in fusicoccadiene synthase (a), iridoid cyclase (b), limonene synthase (c), and cucumene synthase (d). (e) Potential biosynthesis pathway for sesquarterpenes (C35 Terpenes). The heterodimeric heptaprenyl diphosphate synthase (HepS and HepT) converts one farnesyl diphosphate and four isopentenyl diphosphate molecules via a head‐to‐tail condensation reaction to form heptaprenyl diphosphate. This reaction is subsequently followed by cyclization by YtpB and SqhC enzymes, generating one ring at one end of a 35‐carbon chain of heptaprenyl diphosphate and 35‐carbon terpenol with a polycyclic skeleton, respectively. Reproduced from Reference 97. Originally published in Reference 97. Copyright 2011 American Chemical Society
