FIGURE 11.
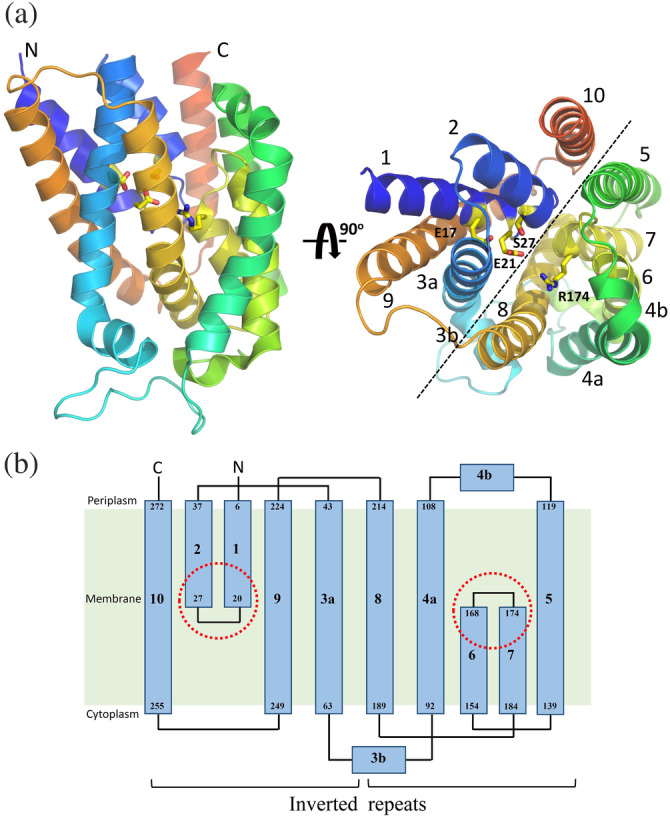
Crystal structure and topology of Escherichia coli undecaprenyl pyrophosphate phosphatase (UPPP). (a) Overall structure of E. coli UPPP. Structural data are available from the PDB file: 5OON or 6CB2. 62 , 63 Ten α‐helices, labeled 1–10, are displayed as a helical cartoon and colored rainbow from the N‐ (blue) to the C‐terminal (red). The orientation of the panel on the right is rotated 90° along the horizontal axis relative to that on the left. The catalytic site residues, Glu‐17, Glu‐21, Ser‐27, and Arg‐174 are presented in yellow and displayed as a stick model. The black dotted line denotes the unique twofold pseudosymmetry axis parallel to the membrane plane. (B) The 10 transmembrane helices are displayed in the two‐dimensional topology of E. coli UPPP to highlight the interlocked inverted repeats. The red dotted circles denote two conserved regions (residues 17–30 and residues 170–178) embedded at the membrane midplane
