Table 1.
Reaction optimization.[a]
|
Entry |
TEMPO (equiv) |
Solvent |
T |
Yield 1 a [%] |
|---|---|---|---|---|
|
1[b] |
5 |
n ‐hexane |
rt |
59 |
|
2[c] |
2 |
n‐hexane |
rt |
35 |
|
3[b] |
10 |
n‐hexane |
rt |
57 |
|
4[b] |
5 |
n‐hexane |
0 °C |
59 |
|
5[b] |
5 |
PhMe |
0 °C |
60 |
|
6 |
5 |
MeCN |
rt |
58 |
|
7 |
5 |
MeCN |
0 °C |
50 |
|
8 |
5 |
MeCN |
−20 °C |
56 |
|
9 |
5 |
1,2‐DCE |
rt |
34 |
|
10[d] |
5 |
MeCN |
70 °C |
30 |
|
11[e] |
5 |
MeCN |
rt |
53 |
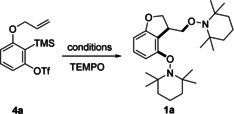
[a] Reaction conditions: 4 a (0.1 mmol, 1.0 equiv), TEMPO (0.5 mmol, 5.0 equiv), CsF (0.3 mmol, 3.0 equiv), and solvent (1 mL, 0.1 m). Yields represent isolated yields. [b] 18‐crown‐6 ether (0.3 mmol, 3.0 equiv) was added. [c] CsF (0.2 mmol, 2.0 equiv) and 18‐crown‐6 ether (0.2 mmol, 2.0 equiv) were used. [d] K2CO3 (0.40 mmol, 4.0 equiv) and 18‐crown‐6 ether (0.40 mmol, 4.0 equiv) were used to prepare the aryne. [e] Tetrabutylammonium difluorotriphenylsilicate (TBAT, 0.30 mmol, 3.0 equiv) was used to prepare the aryne. rt=room temperature.
