Figure 3.
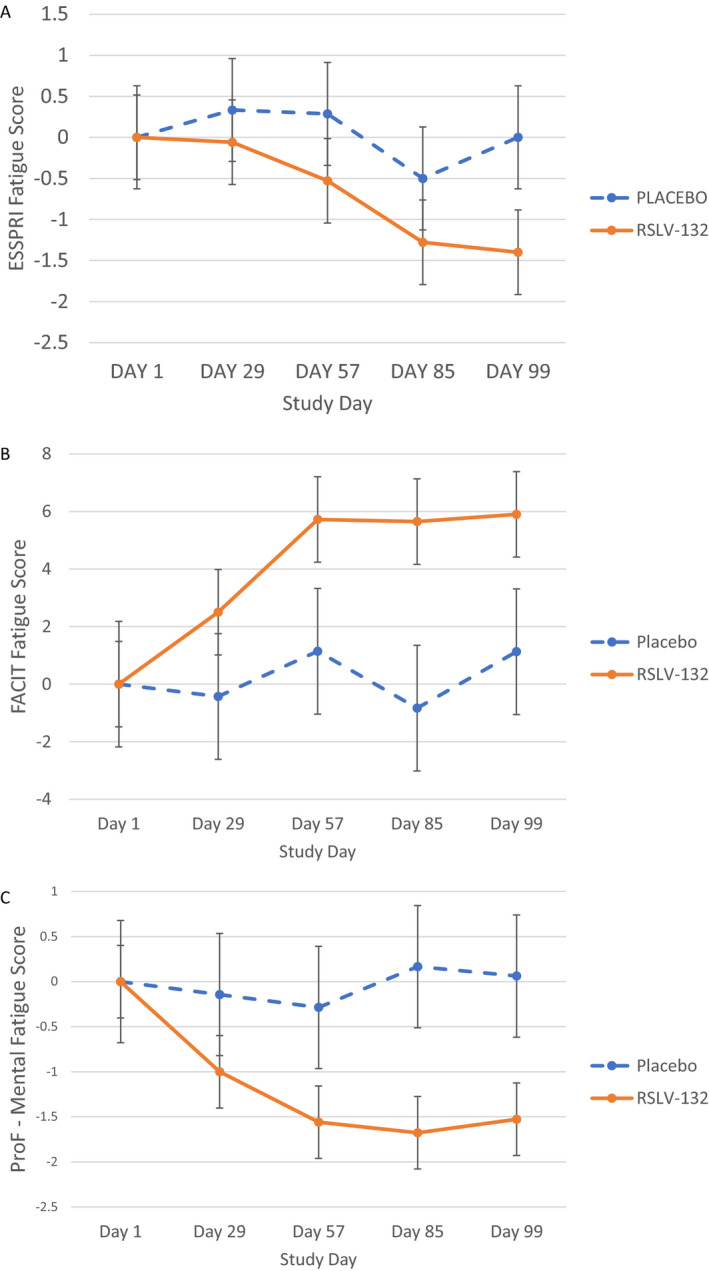
Secondary end point efficacy measures. Clinical efficacy was assessed as the mean change from baseline in the fatigue component of the European League Against Rheumatism Sjögren’s Syndrome Patient Reported Index (ESSPRI) (A), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) (B), and mental fatigue component of the Profile of Fatigue (ProF) (C) in the RSLV‐132 and placebo treatment groups. Groups were compared using separate 1‐way analysis of variance models for each visit, each testing the null hypothesis that the true mean difference between treatment groups was 0 (unadjusted α = 0.05). Between‐group differences were as follows: P = 0.136 in A, P = 0.092 in B, and P = 0.046 in C. Results at each time point are the mean ± SEM.
