ABSTRACT
Our recent genomic studies identified a complex kidney‐specific enhancer module located within the introns of adjacent Mettl1 (M1) and Mettl21b (M21) genes that mediate basal and PTH induction of Cyp27b1, as well as suppression by FGF23 and 1,25‐dihydroxyvitamin D3 [1,25(OH)2D3]. The tissue specificity for this regulatory module appears to be localized exclusively to renal proximal tubules. Gross deletion of these segments in mice has severe consequences on skeletal health, and directly affects Cyp27b1 expression in the kidney. Deletion of both the M1 and M21 submodules together almost completely eliminates basal Cyp27b1 expression in the kidney, creating a renal specific pseudo‐null mouse, resulting in a systemic and skeletal phenotype similar to that of the Cyp27b1‐KO mouse caused by high levels of both 25‐hydroxyvitamin D3 [25(OH)D3] and PTH and depletion of 1,25(OH)2D3. Cyp24a1 levels in the double KO mouse also decrease because of compensatory downregulation of the gene by elevated PTH and reduced FGF23 that is mediated by an intergenic module located downstream of the Cyp24a1 gene. Outside of the kidney in nonrenal target cells (NRTCs), expression of Cyp27b1 in these mutant mice was unaffected. Dietary normalization of calcium, phosphate, PTH, and FGF23 rescues the aberrant phenotype of this mouse and normalizes the skeleton. In addition, both the high levels of 25(OH)D3 were reduced and the low levels of 1,25(OH)2D3 were fully eliminated in these mutant mice as a result of the rescue‐induced normalization of renal Cyp24a1. Thus, these hormone‐regulated enhancers for both Cyp27b1 and Cyp24a1 in the kidney are responsible for the circulating levels of 1,25(OH)2D3 in the blood. The retention of Cyp27b1 and Cyp24a1 expression in NRTCs of these endocrine 1,25(OH)2D3‐deficient mice suggests that this Cyp27b1 pseudo‐null mouse will provide a model for the future exploration of the role of NRTC‐produced 1,25(OH)2D3 in the hormone's diverse noncalcemic actions in both health and disease. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: CYTOCHROME P450; CRISPR/Cas9; ChIP‐seq; VITAMIN D; GENE REGULATION; 1,25(OH)2D3; Cyp27b1‐KO; Cyp24a1; FIBROBLAST GROWTH FACTOR 23; PARATHYROID HORMONE; PTH/Vit D/FGF23; CYTOKINES; TRANSCRIPTIONAL REGULATION; GENETIC ANIMAL MODELS
Introduction
Biological processes integral to the maintenance of mineral homeostasis are highly complex, and likely represent one of the most exquisite regulatory systems that can be defined in higher vertebrates. The need for this regulation is quite clear: Appropriate levels of calcium (Ca) and phosphorus (P), as well as other rare nutrients, are essential for the unique functioning of many if not most life processes. Thus, aberrant levels of these elements can lead to an astounding array of human diseases. Ca and P levels, in particular, are regulated by vitamin D, PTH, and FGF23, the three primary mineralotropic hormones whose independent actions in the intestine, bone, and kidney orchestrate mineral absorption, resorption, and reabsorption, respectively.( 1 ) Interestingly, aside from their unique and frequently overlapping functions in these key tissues, as seen in Fig. 1, each hormone also coordinately regulates the production, processing, and/or activity of the other two.( 2 , 3 , 4 , 5 ) An additional target is the parathyroid gland (PTG) because this organ is the sole producer of PTH. Indeed, PTH is subject to positive regulation by low Ca and negative regulation by FGF23 and 1,25‐dihydroxyvitamin D3 [1,25(OH)2D3] under a variety of physiological states. From a mineral homeostasis perspective, however, the dominant of the three hormones may be vitamin D, given the intricate nature of its metabolic activation, its striking regulation of both PTH and FGF23, and its broad activity profile across tissues. Thus, though each of the three hormones displays novel activities at the kidney and skeleton, and at other nonmineralizing tissues, vitamin D is alone in its capacity to induce dietary Ca and P absorption from the gut.
Fig 1.
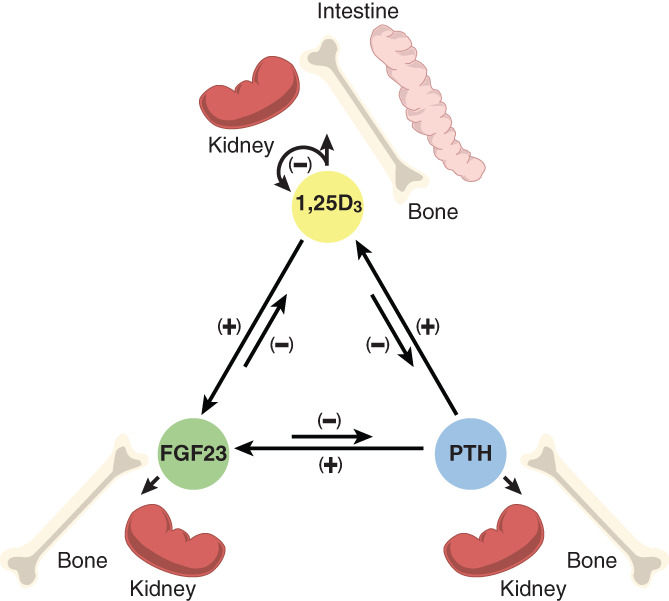
The interregulatory nature of the mineralotropic hormones PTH, FGF23, and 1,25(OH)2D3 (1,25D3) and their genes (via expression of Pth, Fgf23, Cyp27b1, and Cyp24a1). 1,25(OH)2D3 feedback regulates their own expression. Arrows indicate the direction and nature (+/−) of regulation. Kidney, intestine, and bone represent the direct mineral regulating targets of the individual hormones.
Vitamin D is derived through sunlight photo‐conversion from cutaneous 7‐dehydrocholesterol in the skin. Nevertheless, it must be converted via two sequential hydroxylation reactions to the active hormone, first in the liver by CYP2R1 to 25‐hydroxyvitamin D3 [25(OH)D3], though other enzymes and tissues convert smaller amounts, and then in the kidney by CYP27B1 to 1,25(OH)2D3, the active hormone, whereupon it is released as an endocrine principle into the circulation.( 6 ) Of significance, the levels of this hormone are also governed via the regulated expression of Cyp24a1, which initiates the eventual degradation of 1,25(OH)2D3 to calcitroic and calcioic acids via less active 1,24,25(OH)3D3 or 1,23,25(OH)3D3 intermediates.( 6 , 7 ) Thus, 1,25(OH)2D3 levels are determined by the coordinated expression and actions of two gene products; the synthesis and degradation of PTH and FGF23 also involve actions by several different gene products. Recent studies suggest that CYP24A1 also contributes to the regulation of 25(OH)D3 via its actions to maintain appropriate vitamin D substrate concentrations, not through the control of synthesis, but rather through catabolism to the less active metabolites 24,25(OH)2D3 and 23,25(OH)2D3.( 8 ) Thus, the biological function of CYP24A1 may be largely to prevent an inappropriate increase in both 25(OH)D3 and 1,25(OH)2D3 under conditions where these vitamin D metabolites could reach toxic levels, thereby provoking potentially lethal hypercalcemia.
Much of the interregulatory nature of the three mineralotropic hormones as depicted in Fig. 1 has been defined over the past several decades. Thus, it is well known that PTH is a primary inducer of the renal expression of the Cyp27b1 gene encoding CYP27B1 in the kidney, whereas both FGF23 and 1,25(OH)2D3 itself are strong suppressors of Cyp27b1 expression.( 2 , 3 , 4 ) More recent studies have shown that the renal Cyp24a1 gene is transcriptionally regulated reciprocally by these same hormones, driven by homeostatic responses that occur as a result of changes in Cyp27b1 expression that link the actions of both enzymes to the maintenance of appropriate 1,25(OH)2D3 levels.( 8 ) 1,25(OH)2D3 feedback, in turn, downregulates PTH expression/secretion from the PTGs, while simultaneously inducing FGF23 expression from osteocytes in bone.( 9 , 10 ) Like 1,25(OH)2D3, however, FGF23 feedback suppresses the production of PTH, thus providing additional transcriptional control.( 11 ) Importantly, PTH and FGF23 provide direct links to both Ca and P homeostasis, respectively, via the ability of Ca to control PTH and P to control FGF23 levels.
Despite observations over decades documenting the above complex regulatory phenomena, the genomic and molecular mechanisms that mediate these activities in vivo are only now emerging. We turned our attention several years ago toward understanding each of the molecular regulatory events that govern the expression of renal Cyp27b1 and Cyp24a1 and thus the production and maintenance of endocrine 1,25(OH)2D3. The goal was to define the genomic sites of action of each hormone, which we hypothesized would first reveal important initial insights and then provide an entrée into the molecular mechanisms involved. Although we began similar studies of the regulation of PTH and FGF23 genes by these mineralotropic hormones, we summarize in this article our recent efforts to define the genomic mechanisms through which Cyp27b1 and Cyp24a1 expression are regulated by PTH, FGF23, and 1,25(OH)2D3. We took advantage of newly established techniques that enabled an unbiased study of gene regulation entirely in the mouse. Accordingly, we first employed ChIP‐seq analysis of the kidney cortex and other tissues to identify potential sites of genomic action of key transcription factors, to characterize the epigenetic histone environment that surrounded these genomic sites and to determine chromatin/DNA sequence accessibility.( 12 , 13 , 14 , 15 , 16 ) We also extended our findings at Cyp27b1 and Cyp24a1 loci using several genetic mouse models wherein the overall expression of these two genes was strikingly enhanced because of highly elevated PTH and lowered FGF23 levels. Finally, we assessed the functions of these regulatory regions to alter Cyp27b1 expression in vivo by using a CRISPR/Cas9 gene‐editing approach wherein key segments of both genes were deleted individually from the mouse genome and the regulatory, systemic, and skeletal phenotypes evaluated.( 17 , 18 ) Utilizing these techniques, we discovered several complex distal regulatory modules that control the expression of Cyp27b1 and Cyp24a1 uniquely in the kidney that modulate, in turn, the blood levels of endocrine 1,25(OH)2D3.( 8 , 19 , 20 )
Regulation and Maintenance of Vitamin D Metabolism
Identifying the complex tissue‐specific regulatory module that controls renal Cyp27b1 expression and the endocrine production of 1,25(OH)2D3
We commenced our study of the regulation of Cyp27b1 and Cyp24a1 in the kidney by first establishing regulatory responses to exogenous administration of PTH, FGF23, and 1,25(OH)2D3 in vivo. These studies confirmed the reciprocal nature of the response of these two genes to PTH, FGF23, and 1,25(OH)2D3 as previously identified. We then confirmed through gain of function transgene experiments that segments controlling these features of the transcriptional regulation of Cyp27b1 and Cyp24a1 were indeed present and located within the extended genomic regions contained within the transgenes. Accordingly, we introduced large genetically marked BAC clone‐derived segments of DNA into the mouse genome using traditional methods (Fig. 2A ),( 21 , 22 ) and selected gene positive mouse strains that were then explored for their level of basal expression and regulation by PTH, FGF23, and 1,25(OH)2D3. We measured transgene‐derived RNA transcripts using novel probes that required the presence of unique sequences located within the transgenes themselves. As shown in Fig. 2B , examination of the output of both Cyp27b1 and Cyp24a1 genes confirmed appropriate and reciprocal hormonal regulation as previously observed for the same endogenous mouse genes in vivo. The expression of the Cyp24a1 transgene was also assessed in a Cyp24a1‐null mouse following transgenic rescue of Cyp24a1 expression in this Cyp24a1‐null mouse via a genetic cross. Transgenic expression of Cyp24a1 in the kidneys of the rescued mouse resulted in the appearance of 24,25(OH)2D3 in the blood at levels slightly higher than those seen in normal animals.( 8 ) These levels were caused by a below‐normal expression of the Cyp24a1 transgene in the kidneys that resulted in a paradoxical rise in 24,25(OH)2D3 as previously discussed.( 8 ) This transgenic confirmation of regulation within defined Cyp27b1 and Cyp24a1 loci narrowed the potential location of regulatory elements and was necessary in light of recent genomic discoveries indicating that regulatory regions for genes can occur frequently many kilobases or even megabases distal to their genetic targets.( 16 ) Our transgenic results provided the rationale for a more‐focused search for genomic elements within the surrounding loci for both Cyp27b1 and Cyp24a1 that could regulate the expression of both genes in the kidney.
Fig 2.
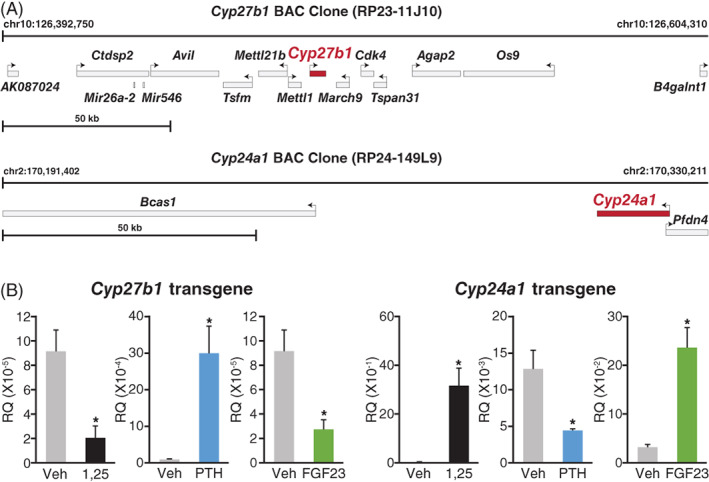
Extended bacterial artificial chromosome (BAC) clone transgenes that contain mouse Cyp27b1 and Cyp24a1 genetic loci recapitulate the hormonal regulation by exogenous PTH, FGF23, and 1,25(OH)2D3 regulation seen for endogenous genes in mice. (A) Schematic depiction of mouse transgene structures. (B) Hormonal regulation. Transgenic mice were prepared and selected as recently reported.( 22 ) Animals were injected with PTH (230 ng/g body weight [BW]; blue), FGF23 (50 ng/g BW; green), or 1,25(OH)2D3 (10 ng/g BW; black) and tissues harvested at 1, 3, and 6 hours, respectively. Transgene‐derived Cyp27b1 and Cyp24a1 transcripts were quantitated using probes that required the presence of the internal ribosome entry‐site module incorporated into the BAC clones. Data are derived from four to six mice per group, and presented as the means ± SEM, *p < 0.05.
Identifying the sites of action of PTH, FGF23, and 1,25(OH)2D at the Cyp27b1 gene in the kidney
As indicated above, we utilized ChIP‐seq analysis of the kidney to identify the potential sites of action of each hormone at the Cyp27b1 and Cyp24a1 loci.( 19 ) An initial examination following injection of 1,25(OH)2D3 or PTH revealed the presence of four novel vitamin D receptor (VDR)‐bound sites located within the introns of the immediately upstream genes Mettl1 and Mettl21b (now termed Eef1akmt3), sites that we designated M1 and M21 as identified in Fig. 3.( 19 ) A known mediator of PTH action via the PKA pathway, p133‐CREB (pCREB) also colocalized to these sites. Exploration of the histone environment surrounding these sites also revealed the presence of histone marks consistent with epigenetic characteristics of regulatory elements.( 23 , 24 ) These modifications included H3K4 methylation (me1), H3K9 acetylation, and H3K36 methylation (me3). Importantly, ChIP‐seq analyses of the changes that occurred to these histone marks upon injection of PTH, 1,25(OH)2D3, and FGF23 were indicative of altered gene expression, strongly indicating that these regulatory regions were active. PTH mediated an upregulation of Cyp27b1, whereas FGF23 and 1,25(OH)2D3 mediated suppression. Thus, although PTH is known to activate a number of transcription factors in addition to pCREB, the presence of the VDR and pCREB suggested that 1,25(OH)2D3 and PTH were active at these four sites. In the case of FGF23, however, because the transcription factor pathways for this hormone at Cyp27b1 and Cyp24a1 are currently unknown, only increased epigenetic histone activity pointed to where this hormone might act. We also discovered that each of these novel sites contained an open chromatin configuration, which is essential to the functional operation of genomic control elements, an experimental result conducted in the kidney by the ENCODE (Encyclopedia of DNA Elements) Consortium via DNase hypersensitivity sequencing (DHS)‐based analysis.( 16 ) Interestingly, none of these features was present within the Mettl1 and Mettl21b genes in any nonrenal tissues. This finding supports our conclusion that the regulatory module we identified is likely specific to the kidney and represents the sole determinant of unique Cyp27b1 response to PTH, FGF23, and 1,25(OH)2D3 that links the endocrine production of 1,25(OH)2D3 to Ca and P homeostasis.
Fig 3.
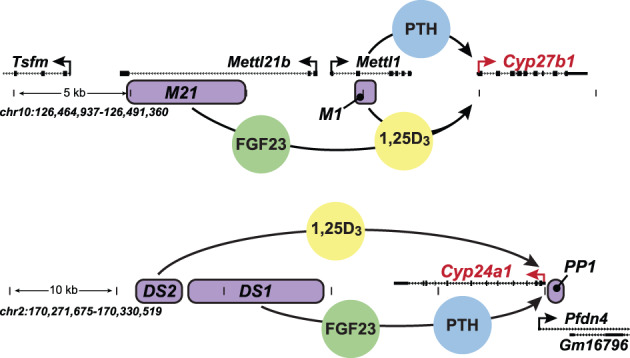
Schematic representation of the genomic enhancers for Cyp27b1 and Cyp24a1. (A) The locations of enhancers for Cyp27b1 are shown in purple and designated M1 and M21 with the gene dense region of the Cyp27b1 locus, and mediated by PTH (M1; blue), FGF23 (M21; green), and 1,25(OH)2D3 (M1 and M21; yellow). (B) The locations of enhancers for Cyp24a1 are shown in purple and designated PP1, DS1, and DS2 within the Cyp24a1 gene locus, and mediated by PTH and FGF23 (DS1; blue and green) and 1,25(OH)2D3 (PP1 and DS2; yellow). Figure modified from Meyer and Pike.( 26 )
Identifying the sites of action of PTH, FGF23, and 1,25(OH)2D 3 at the Cyp24a1 gene in the kidney
With regard to the reciprocal regulation of Cyp24a1 by PTH, FGF23, and 1,25(OH)2D3, as depicted in Fig. 3, ChIP‐seq analysis of kidney DNA surrounding this gene's locus in mice revealed a similar overall organization.( 8 ) Thus, two extended, but separate regions downstream of the gene were observed that, in addition to well‐known promoter‐proximal sites, bound occupied clusters of either VDR or pCREB following either 1,25(OH)2D3 or PTH injection. Our earlier studies of nonrenal cells in vitro had indicated that one of these downstream regions bound the VDR and contained vitamin D response elements that were transcriptionally active at the Cyp24a1 promoter.( 25 ) Further analysis of the kidney revealed that the chromatin state and epigenetic histone environment across these downstream regions in the kidney were also characteristic of regulatory regions and that the appropriate reciprocal regulation of epigenetic histone density was exerted by PTH and 1,25(OH)2D3 as well, indicating that these regions were transcriptionally active. Importantly, regulation of the density of these histone marks by FGF23 exclusively within the region that also bound pCREB provided unique support for a direct role for this hormone's induction of Cyp24a1 expression, thereby providing a potential mechanistic linkage between the opposing regulatory actions of PTH and FGF23 at this gene. Interestingly, analogous to Cyp27b1, this downstream chromatin feature present at the PTH and FGF23 sensitive region in Cyp24a1 locus is absent in nonrenal tissues. This provides a mechanistic explanation for why the actions of PTH and FGF23 at the Cyp24a1 gene appear to be limited to the kidney, whereas those of 1,25(OH)2D3 itself span all tissues that express the VDR.
Characterizing the regulatory phenotypes of mice with mutations in the Cyp27b1 regulatory module
The identification of potential sites of action of PTH, FGF23, and 1,25(OH)2D3 at the Cyp27b1 and Cyp24a1 genes prompted a series of loss of function studies to characterize the specific activities of these regulatory modules at Cyp27b1 and Cyp24a1 in vivo and to confirm whether these modules were indeed functionally specific for the kidney.( 19 , 20 , 26 ) CRISPR/Cas9‐mediated gene‐editing techniques in mice provided the essential tool through which we could examine the potential functional features of these regulatory regions across multiple tissues, including the kidney. The editing technique also provided the opportunity to evaluate the phenotypic consequences that emerged following the alteration of Cyp27b1 and Cyp24a1 expression via these regulatory deletions, including an assessment of consequences on the production of endocrine 1,25(OH)2D3 itself. As shown in Fig. 3, we utilized pairs of RNA guides to direct Cas9 digestions in oocytes and created mice whose genomes contained an approximate 400‐bp deletion (M1) within the intron of Mettl1 (termed M1‐IKO for M1‐intronic knockout), a 5‐kb deletion (M21) within the extended intron of Mettl21b (termed M21‐IKO) and a double deletion at both M1 and M21 (termed M1/M21‐DIKO). Although all three strains were indistinguishable from WT littermates at weaning, M1‐IKO and M1/M21‐DIKO mice began to exhibit retarded growth patterns early on that resulted in reduced body weight and smaller stature, which by 8 weeks reflected the physical appearance of Cyp27b1‐null mice. In contrast, the growth pattern and the physical appearance of the M21‐IKO mice were unremarkable relative to their WT littermate counterparts. Systemic measurements of Ca, P, PTH, and FGF23 revealed that like those of the Cyp27b1‐null mouse, both M1‐IKO and M1/M21‐DIKO mice exhibited hypocalcemia, hypophosphatemia, hyperparathyroidism, and very low levels of FGF23, all indicative of a potential reduction or the absence of circulating 1,25(OH)2D3. Indeed, measurements of the vitamin D hormone in the blood revealed substantially lower, but not absent levels in M1‐IKO mice and even lower levels in the M1/M21‐DIKO mice; these levels were undetectable in Cyp27b1‐null mice. As with the latter mice, however, both the M1 and M1/M21 deleted strains also exhibited striking changes in skeletal morphology and low BMD as previously identified. These additional systemic, hormonal, or skeletal features were absent in the M21‐IKO mice.
The underlying molecular basis for these phenotypic differences between M1‐IKO and M1/M21‐DIKO mice and M21‐IKO and WT mice emerged upon analysis of the expression patterns of Cyp27b1 and Cyp24a1 in the kidney. Accordingly, Cyp27b1 expression was reduced dramatically in M1‐IKO and M1/M21‐DIKO mice; the latter strain retained a 99% reduction. A similar reduction in the level of renal Cyp24a1 expression was also noted, a decrease that correlated directly with the absence of circulating 24,25(OH)2D3. Interestingly, although M21‐IKO mice also exhibited reductions in renal Cyp27b1 and Cyp24a1 expression, these decreases were not as profound. Unexpectedly, however, circulating 1,25(OH)2D3 and 24,25(OH)2D3 were either normal or slightly above normal, respectively. The striking reduction in Cyp24a1 expression in M1‐IKO and M1/M21‐DIKO mice was also accompanied by extremely high levels of 25(OH)D3 relative to WT and M21‐IKO mice as well. Finally, despite the overall impact of these deletions on Cyp27b1 and Cyp24a1 expression in the kidney and on the phenotype of each of these mutant mouse strains, there was no effect observed on the basal expression of these two genes in nonrenal tissues such as skin, bone, intestine, spleen, or immune cells.
Characterizing the regulatory phenotypes of mice with mutations in the Cyp24a1 regulatory module
We also utilized the CRISPR/Cas9 approach to delete the separate regulatory regions located downstream of the Cyp24a1 gene that bound clusters of either VDR or CREB, as illustrated in Fig. 3 and discussed above, and that appeared to mediate the expression of Cyp24a1 in the kidney.( 8 ) Deletion of the DS1 region that mediated both the downregulation of Cyp24a1 by PTH and its upregulation by FGF23 resulted in a significant reduction in basal Cyp24a1 expression and complete loss of response to both hormones in the kidney, but no reduction in renal response to 1,25(OH)2D3. 1,25(OH)2D3 activity was also retained in nonrenal tissues where, as expected, PTH or FGF23 were inactive. Interestingly, deletion of the DS2 region that mediated the downstream actions of 1,25(OH)2D3 on Cyp24a1 expression had no effect on the gene's basal expression or on its suppression by PTH in the kidney or its induction by either FGF23 or 1,25(OH)2D3. Surprisingly, however, deletion of this region decreased the efficacy of response to 1,25(OH)2D3 in nonrenal tissues such as intestine and bone. No striking phenotypic alterations were identified in either DS1‐KO or DS2‐KO mice with the exception that the loss of basal expression of Cyp24a1 in the DS1 strain resulted in modest homeostatic compensatory changes in PTH and FGF23 levels and a reduction in the renal expression of Cyp27b1. This feature reinforces the idea of the reciprocal coregulation of Cyp27b1 and Cyp24a1 in the kidney that serves to maintain levels of circulating 1,25(OH)2D3. Therefore, our results support the observation at chromatin‐, epigenetic‐, and now gene‐regulatory levels that the downstream PTH/FGF23 regulatory module is active only in the kidney, thereby defining an underlying mechanism through which homeostatic control of Cyp24a1 is restricted to this organ. However, it also illuminates a novel finding that the vitamin D‐regulated module downstream of the gene is also dispensable in the kidney, providing a focus on promoter‐proximal vitamin D regulatory elements, but enhances the sensitivity of induction of Cyp24a1 by 1,25(OH)2D3 in nonrenal tissues. Thus, the coordinated expression of both Cyp27b1 and Cyp24a1 to maintain circulating 25(OH)D3 and 1,25(OH)2D3 levels in vivo is determined by common structural features within chromatin that facilitate the differential expression and regulation of Cyp27b1 and Cyp24a1 in either the kidney or in nonrenal tissues.
Cyp27b1 and Cyp24a1 genes are regulated and functional in renal proximal tubules
Although early studies suggested that Cyp27b1 and Cyp24a1 were expressed selectively in the proximal tubules of the kidney, respectively, more recent studies using immunocytochemical analyses have indicated that these genes, and especially Cyp27b1, could be produced in additional renal cell types, as well as other nonrenal cell types.( 27 , 28 , 29 , 30 , 31 ) This uncertainty drove our initial decision to explore the entire kidney as above, yet represented a potential complexity relevant to the interpretation of our initial genomic studies of Cyp27b1 expression. This issue also raised additional biological questions relative to the linkage between Cyp27b1 and Cyp24a1. Fortunately, however, recent genomic studies have been conducted by Cusanovich and colleagues on individual kidney cell isolates, as well as numerous nonrenal cell types from C57BL/6 mice using ATAC‐seq analysis.( 32 ) This approach, like DHS, reveals the presence of open chromatin sites across genomes.( 33 , 34 , 35 ) Several genomic data tracks from these analyses at the Cyp27b1 and Cyp24a1 gene loci are documented in Fig. 4. As can be seen, these analyses reveal that the regulatory sites with open chromatin features that we defined within the introns of Mettl1 and Mettl21b in total kidney tissues are also evident exclusively in cells of proximal, but not distal tubule origin, or indeed in other cells of either renal or nonrenal origin. These results confirm that proximal tubules are the principle sites of Cyp27b1 expression and the dominant source for the regulated production of endocrine 1,25(OH)2D3. Indeed, they confirm that the original identification of proximal tubules as the sources of Cyp27b1 expression and the production of 1,25(OH)2D3 was likely correct. These data also indicate that the regulatory module we identified previously is not just kidney‐specific, but rather proximal renal tubule‐specific. Whether Cyp27b1 expression occurs that is insensitive to PTH and FGF23 regulation in additional kidney cell types, consistent with immunocytochemical identification, remains to be resolved. Interestingly, the open chromatin states as seen in Fig. 4 that define the regulatory expression of Cyp24a1 in the kidney are also present exclusively in proximal, but not distal tubules or the other renal cell types, as previously suggested. In conclusion, Cyp27b1 and Cyp24a1 are both expressed in the same key endocrine cell type in kidney, and thus capable of coordinating in real time the coregulation of endocrine 1,25(OH)2D3 production that is subsequently secreted into the blood. This has profound implications for the importance of both genes as determinants of vitamin D activation and maintenance, illustrating the power of genomic approaches for illuminating key physiological principles.
Fig 4.
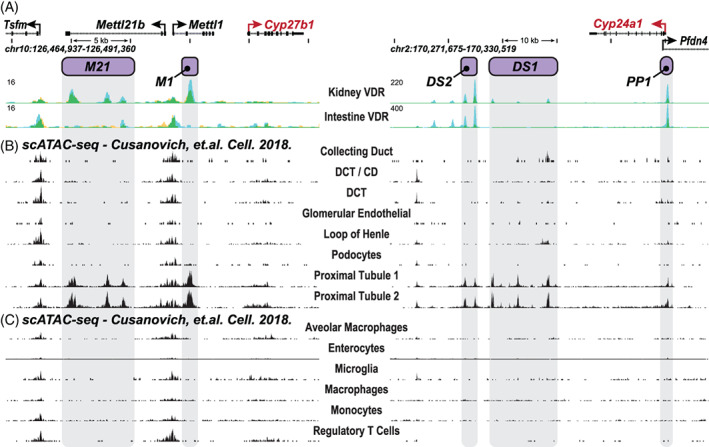
Single cell genome analyses of chromatin accessibility in renal and nonrenal cell types in the mouse. (A) ChIP‐seq analysis of VDR binding at the kidney cortex and intestine documenting the locations of the regulatory submodules (gray‐shaded dropdown bars) that mediate regulation by PTH (M1; purple) and FGF23 (M21, purple) as well as 1,25(OH)2D3 (M1 and M21). Data from Meyer et al.( 8 , 19 , 20 ) The upper panel indicates the chromosomal location of Cyp27b1 and Cyp24a1, genome scale, and the identity of adjacent genes. Arrows indicate the direction of gene transcription. As seen, the overall module is restricted to the kidney, where it is dispersed within single introns of the Mettl1 and Mettl21b genes, and absent in intestine (and all other tissues; see 8, 19, 20 )). (B,C) Single‐cell ATAC‐seq (scATAC‐seq) analysis by Cusanovich and colleagues.( 32 ) Raw data were reanalyzed and displayed for only the Cyp27b1 and Cyp24a1 gene loci for (B) ATAC‐seq analysis of individual renal cell types and (C) analysis of representative nonrenal cell types (emphasis on immune cell types). As seen, peaks representative of an open chromatin state align with each of the individual components that comprise the kidney‐specific module (M1 and M21) and are restricted to renal proximal tubules (two separate analyses). No other renal or nonrenal cells types retain this chromatin regulatory pattern.
Creation of a kidney‐specific Cyp27b1 pseudo‐null mouse deficient in endocrine 1,25(OH)2D3
Research over the past several decades has suggested that the conversion of 25(OH)D3 to 1,25(OH)2D3 occurs not only in the kidney, as above, but also in a myriad of nonrenal tissues/cells (NRTCs) that include the skin, parathyroid glands, bone cells, both cardiovascular and immune cells, and many others.( 30 , 36 , 37 ) This idea stems from early immunocytochemical observations suggesting that CYP27B1 expression is also present in NRTCs, albeit at very low levels relative to the primary renal source. It also became evident that the regulation of Cyp27b1 expression in NRTCs is different from that in the kidney. Accordingly, though renal Cyp27b1 expression is tightly modulated by PTH, 1,25(OH)2D3, and FGF23, and now known to occur through the proximal tubule‐specific renal regulatory module described earlier, these mineralotropic hormones are generally inactive at Cyp27b1 in NRTCs, where inflammatory mediators such as IL‐1β, TNFα, LPS, and certainly others function to induce this gene.( 38 ) Some insight has emerged with regard to the pathways that are involved in Cyp27b1 induction by these inflammatory modulators, although the sites of action of these regulators at Cyp27b1 itself have not been identified in vivo. Moreover, although it is now clear why Cyp27b1 is regulated in the kidney, but not in NRTCs by the three mineralotropic hormones, the molecular basis for the differential basal expression of Cyp27b1 expression in the kidney and NRTCs, although under investigation, remains unknown. Elucidation of this feature is important because it is likely integral to the evolutionary development and maintenance of the vitamin D endocrine system.
Advancing the features of Cyp27b1 expression and activity in NRTCs
Aside from the concept of local production of 1,25(OH)2D3, it is noteworthy that although the synthesis of 1,25(OH)2D3 in NRTCs has gained wide acceptance, fundamental insights supporting the mechanism, relevance, and biological consequence of this cellular source of 1,25(OH)2D3 production remain outstanding. Importantly, although attempts have been made to selectively delete Cyp27b1 from specific cell types such as chondrocytes, and to assess the phenotypic consequences of these gene deletions on specific tissues, this approach has met with only modest success, perhaps because fundamental questions regarding NRTC production of 1,25(OH)2D3 in the absence of renal production remain at issue.( 39 ) Key issues pertinent to the nature of local production are as follows: (i) Does 1,25(OH)2D3 production in NRTCs occur in healthy as well as in diseased subjects in vivo, and does this local synthesis exert a measurable impact on the mechanisms of vitamin D receptor activation that both selectively alter gene expression and uniquely modify the functions of the individual cell types involved; (ii) is locally produced 1,25(OH)2D3 routinely secreted into the blood in health as has been amply demonstrated in certain human disease states; (iii) is the overall activity of locally produced 1,25(OH)2D3 influenced by or dependent upon the circulating levels of endocrine‐derived hormone; and (iv) does vitamin D supplementation and/or circulating concentrations of substrate 25(OH)D3 differentially impact the local versus kidney production of 1,25(OH)2D3. Resolution of these and other issues may lie at the heart of a successful vitamin D supplementation regimen capable of achieving effective therapeutic efficacy in the prevention or treatment of disease. It is noteworthy, however, that precedent has been firmly established in humans for both the production and secretion of 1,25(OH)2D3 from macrophages derived from patients with a diverse set of granulomatous diseases.( 40 , 41 ) The elevated levels of 1,25(OH)2D3 are indeed active in these patients and exaggerate the disease by accelerating the uptake of calcium from the gut that results in hypercalcemia. Nevertheless, the overall relationship between this specific NRTC activity to produce 1,25(OH)2D3 relative to that derived from the kidney remains to be fully understood. Indeed, not all patients with inflammatory diseases present with elevated blood levels of 1,25(OH)2D3 and hypercalcemia.
Utility of animal models to explore NRTC expression and regulation of Cyp27b1
It is clear that unique animal models selectively deficient in the endocrine production of 1,25(OH)2D3 will be essential for advancing our understanding of whether and how the local production of 1,25(OH)2D3 is achieved and how it contributes to biology. These models will have to be amenable to exploration into the specific issues outlined above and, in particular, to the genetic imposition of inflammatory disease states such as CKD, IBD, atherosclerosis, or perhaps even infectious diseases such as COVID‐19. Models such as the latter will enable an evaluation of the hypothesis that 1,25(OH)2D3 production is accelerated through an inflammation‐induced upregulation of Cyp27b1 expression in the immune system, but not in kidney, for example, that will reduce the overall state of inflammation. Unfortunately, global Cyp27b1‐null mice are inappropriate, and efforts to create a CRE‐generated kidney‐selective Cyp27b1‐null mouse have thus far been unsuccessful largely because of the complexity of distinct cell types that comprise even renal proximal tubules. Regardless of whether this is achieved, however, the linkage between renal Cyp27b1 and Cyp24a1 expression suggests that any downregulation of Cyp27b1 will be accompanied by a similar suppression of Cyp24a1 expression, thereby preserving even small amounts of 1,25(OH)2D3 that might be secreted into the blood, although not sufficiently active.( 8 , 19 , 20 ) Indeed, it is clear that Cyp27b1 expression is unlikely to be genetically modified and/or suppressed without the homeostatic downregulation of Cyp24a1 by PTH and FGF23.
The Cyp27b1 pseudo‐null M1/M21‐DIKO mouse as a model for studying NRTC production of 1,25(OH)2D3
Interestingly, the M1/M21‐DIKO mouse described above exhibits just such a Cyp27b1 pseudo‐null phenotype wherein the production of 1,25(OH)2D3 is strongly downregulated and incapable of maintaining normal PTH and FGF23 balance and mineral homeostasis.( 20 ) This state profoundly disrupts skeletal development and integrity, and mimics the overall Cyp27b1‐null mouse phenotype. Given the suppression and loss of regulation of Cyp27b1 expression, it was surprising that even low detectable levels of 1,25(OH)2D3 were evident in the blood. Our interpretation of this finding is that though well below normal, the circulating levels of the kidney‐derived hormone still remain “inappropriately high” because of the striking suppression of renal Cyp24a1 expression and 24,25(OH)2D3 production that is evident as a result in high PTH and low FGF23 levels. This linkage to Cyp24a1 expression as suggested earlier, however, resulted in the absence of both 1,25(OH)2D3 and 25(OH)D3 catabolism in the kidney, thus raising both of these vitamin D metabolites to higher than expected levels in the blood. Given the inability of the mutated Cyp27b1 gene in these mice to respond to PTH, FGF23, or 1,25(OH)2D3 regulation, we hypothesized that renal Cyp24a1 levels under dietary conditions of high Ca and P exposure should be upregulated and restored as a result of the normalization of PTH and FGF23 levels, analogous to that seen in Cyp27b1‐null mice. Indeed, the application of this “rescue” diet to these M1/M21‐DIKO mice fully normalized the aberrant levels of high PTH and low FGF23, appropriately raised Cyp24a1 expression without an effect on Cyp27b1, dramatically reduced 25(OH)D3 levels, and fully eliminated 1,25(OH)2D3 in the blood, as measured in the latter two cases by liquid chromatography–tandem mass spectrometry analysis. This diet also restored all the systemic parameters of normal mineral metabolism, induced genes essential for Ca and P uptake in the intestine, and rescued the skeletal phenotype as well. Cyp27b1 and Cyp24a1 expression in all NRTCs was unperturbed, as were genes that might be expressed as a result of the local production of 1,25(OH)2D3. These observations did not support the alternative explanation that the modest amounts of circulating 1,25(OH)2D3 in the M1/M21‐DIKO mouse were caused by the induced secretion of 1,25(OH)2D3 from NRTCs. We conclude that this rescued mouse strain, amenable to additional dietary and genetic as well as disease‐inducing manipulations, will likely prove useful in exploring key details of the NRTC production of 1,25(OH)2D3.
Summary and Conclusions
Here we have summarized our recent work utilizing a series of genomic approaches coupled with loss‐of‐function studies, which has identified novel distal regulatory modules that mediate the reciprocal expression of Cyp27b1 and Cyp24a1 in the kidneys of mice. This dual regulation is essential for the control of endocrine 1,25(OH)2D3 in the circulation. Additional studies suggest that the regulated expression of both genes by these modules occurs exclusively in proximal tubules. The renal module for Cyp27b1 is dispersed and located within specific introns in the adjacent Mettl1 and Mettl21b genes in the kidney and is absent in all NRTCs. The submodule in Mettl1 controls the upregulation of Cyp27b1 by PTH, whereas three separate submodules in the Mettl21b gene control suppression by FGF23. All four submodules mediate downregulation by 1,25(OH)2D3. Thus, 1,25(OH)2D3 functions to reinforce the suppressive regulatory actions of FGF23, while opposing the inducing actions of PTH at the Cyp27b1 gene. Of course, 1,25(OH)2D3 also induces Cyp24a1 as well as Fgf23.
The renal module for Cyp24a1 regulation is located intergenically downstream of the gene and is comprised of one segment that mediates opposing regulation by PTH and FGF23. Although this module is absent in NRTCs, a second module, which controls positive regulation by 1,25(OH)2D3, is present and active in all cell types that are targets of 1,25(OH)2D3 activity except the kidney. Deletion of the PTH sensitive component (M1) in the Cyp27b1 gene or both components simultaneously (M1/M21‐DIKO) lead to a decrease in basal expression of Cyp27b1 of up to 99%, and strongly reduces circulating levels of endocrine 1,25(OH)2D3. Lowered basal levels of 1,25(OH)2D3 in M1‐IKO and M1/M21‐DIKO mice lead, in turn, to reduced intestinal absorption of Ca and P, which causes hypocalcemia and hypophosphatemia that promotes a rise in PTH and a reduction in FGF23 levels, and a broad Cyp27b1 null‐like skeletal phenotype. Cyp24a1, on the other hand, is downregulated by these high PTH and low FGF23 levels, which leads to high levels of 25(OH)D3 and a loss of 24,25(OH)2D3. Both features are responsible for the low, residual levels of 1,25(OH)2D3 in the M1‐IKO and M1/M21‐DIKO mice.
Rescue of these mice with high Ca and P diets, particularly the M1/M21‐DIKO mice, raises systemic Ca, P, and FGF23; suppresses PTH; and normalizes the expression of Cyp24a1. This homeostatic increase reduces 25(OH)D3 levels and eliminates circulating 1,25(OH)2D3. These observations provide supportive evidence for our conclusion that Cyp27b1 and Cyp24a1 are coregulated in the kidney, and that the residual source of 1,25(OH)2D3 in the blood of M1/M21‐DIKO mice on a normal mineral diet is not derived from NRTC sources, but rather from the kidney. Diet‐rescued M1/M21‐DIKO mice, devoid of Cyp27b1 expression and circulating endocrine 1,25(OH)2D3, but with intact expression and regulation of Cyp27b1 in NRTC, represent an appropriate model with which to explore features of Cyp27b1 expression in NRTC. These include its mechanisms, substrate dependencies, role in NRTC production of 1,25(OH)2D3, and impact on noncalcemic actions in multiple tissues in both healthy subjects and in disease. Rescued M1/M21 mutant mice will be useful for exploring supplementation, the impact of 25(OH)D3 on Cyp27b1 expression in renal and nonrenal tissues, and the influence of endocrine 1,25(OH)2D3 on local 1,25(OH)2D3 activation of gene expression.
A collection of previous gene deletion models has enhanced our understanding of both vitamin D metabolism and mineral homeostasis. These individual models have been placed strategically in the context of the actions of the three mineralotropic hormones or their mediators in Fig. 5. We have now added at the appropriate interaction nodes, our newly defined mouse regulatory deletion models, providing an additional level of mechanistic complexity to the interactions that occur between the three hormones to regulate vitamin D metabolism and ultimately mineral homeostasis. Our current studies are now focused on the molecular details of the renal regulation of Cyp27b1 and Cyp24a1 and the genomic mechanisms through which the nonrenal expression of Cyp27b1 activity is achieved. They are also aimed at exploiting the Cyp27b1 pseudo‐null mouse to study the nonrenal regulation of Cyp27b1 expression and to determine the underlying genomic mechanisms through which this is achieved.
Fig 5.
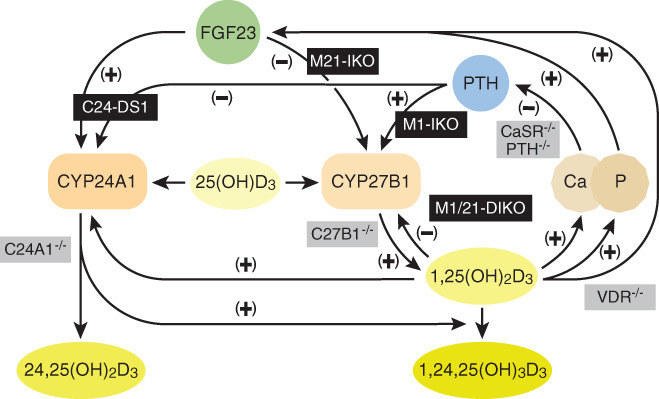
Vitamin D metabolism in the kidney. Schematic diagram depicting the regulation of vitamin D metabolism and serum calcium and phosphate homeostasis in the kidney. Our genetic models (black) and previously existing models (gray) are overlaid on or near the pathways they disrupt. Figure modified from Meyer and Pike.( 26 )
Disclosures
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
J. Pike: Conceptualization; funding acquisition; project administration; supervision; writing‐original draft; writing‐review and editing. Seong min Lee: Data curation; investigation; methodology. Nancy Benkusky: Data curation; investigation; methodology. Mark Meyer: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; writing‐original draft; writing‐review and editing.
Authors' roles
Study design: JWP and MBM. Data collection: MBM, SML, and NAB. Data analysis: MBM, SML, and NAB. Manuscript drafting and revisions: JWP and MBM. Approving final manuscript: JWP, SML, NAB, and MBM.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jbm4.10433.
Acknowledgments
This work was supported by the Department of Biochemistry, University of Wisconsin–Madison, University of Wisconsin Carbone Cancer Center Support Grant P30, and by NIH‐NIDDK award R01‐DK117475 to JWP. We thank members of the Pike Laboratory for helpful discussions and Kathy Krentz in the University of Wisconsin Biotechnology Center–Genome Editing and Animal Models Core for generating the CRISPR/Cas9 enhancer‐deleted mice. We also thank our collaborators Drs. Martin Kaufmann and Glenville Jones at Queen's University for their exhaustive vitamin D metabolite measurements and interpretations that have proven crucial to our understanding of these animals and mechanisms. We also thank Dr. Rene St Arnaud for providing the Cyp24a1 KO mouse and Alex Carlson for assistance in preparing and evaluating the Cyp24a1/transgenic rescue mouse.
REFERENCES
- 1. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 suppl):1689S–96S. [DOI] [PubMed] [Google Scholar]
- 2. DeLuca HF. Parathyroid hormone as a trophic hormone for 1,25‐dihydroxyvitamin D3, the metabolically active form of vitamin D. N Engl J Med. 1972;287(5):250–1. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka Y, DeLuca H. Rat renal 25‐hydroxyvitamin D3 1‐ and 24‐hydroxylases: their in vivo regulation. Am J Physiol. 1984;246(2 pt 1):E168–73. [DOI] [PubMed] [Google Scholar]
- 4. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF‐23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35. [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones G, Prosser DE, Kaufmann M. Cytochrome P450‐mediated metabolism of vitamin D. J Lipid Res. 2014;55(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaufmann M, Martineau C, Arabian A, Traynor M, St‐Arnaud R, Jones G. Calcioic acid: in vivo detection and quantification of the terminal C24‐oxidation product of 25‐hydroxyvitamin D. J Steroid Biochem Mol Biol. 2019;188:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer MB, Lee SM, Carlson AH, et al. A chromatin‐based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non‐renal tissues. J Biol Chem. 2019;294(39):14467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silver J, Moallem E, Kilav R, Sela A, Naveh‐Many T. Regulation of the parathyroid hormone gene by calcium, phosphate and 1,25‐dihydroxyvitamin D. Nephrol Dial Transplant. 1998;13(suppl 1):40–4. [DOI] [PubMed] [Google Scholar]
- 10. Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318(9):1040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mace ML, Gravesen E, Nordholm A, et al. Kidney fibroblast growth factor 23 does not contribute to elevation of its circulating levels in uremia. Kidney Int. 2017;92(1):165–78. [DOI] [PubMed] [Google Scholar]
- 12. Meyer MB, Benkusky NA, Lee CH, Pike JW. Genomic determinants of gene regulation by 1,25‐dihydroxyvitamin D3 during osteoblast‐lineage cell differentiation. J Biol Chem. 2014;289(28):19539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer MB, Benkusky NA, Pike JW. Profiling histone modifications by chromatin immunoprecipitation coupled to deep sequencing in skeletal cells. Methods Mol Biol. 2015;1226:61–70. [DOI] [PubMed] [Google Scholar]
- 14. Turner BM. Defining an epigenetic code. Nat Cell Biol. 2007;9(1):2–6. [DOI] [PubMed] [Google Scholar]
- 15. Ramsey S, Knijnenburg T, Kennedy K, et al. Genome‐wide histone acetylation data improve prediction of mammalian transcription factor binding sites. Bioinformatics. 2010;26(17):2071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stamatoyannopoulos JA, Snyder M, Hardison R, et al. An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biol. 2012;13(8):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sashital DG, Wiedenheft B, Doudna JA. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol Cell. 2012;46(5):606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Yang H, Shivalila CS, et al. One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell. 2013;153(4):910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer MB, Benkusky NA, Kaufmann M, et al. A kidney‐specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J Biol Chem. 2017;292(42):17541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer MB, Benkusky NA, Kaufmann M, et al. Targeted genomic deletions identify diverse enhancer functions and generate a kidney‐specific, endocrine‐deficient Cyp27b1 pseudo‐null mouse. J Biol Chem. 2019;294(24):9518–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onal M, Bishop KA, St John HC, et al. A DNA segment spanning the mouse Tnfsf11 transcription unit and its upstream regulatory domain rescues the pleiotropic biologic phenotype of the RANKL null mouse. J Bone Miner Res. 2015;30(5):855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SM, Bishop KA, Goellner JJ, O'Brien CA, Pike JW. Mouse and human BAC transgenes recapitulate tissue‐specific expression of the vitamin D receptor in mice and rescue the VDR‐null phenotype. Endocrinology. 2014;155(6):2064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pike JW, Meyer MB, St John HC, Benkusky NA. Epigenetic histone modifications and master regulators as determinants of context dependent nuclear receptor activity in bone cells. Bone. 2015;81:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ernst J, Kheradpour P, Mikkelsen TS, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25‐dihydroxyvitamin D3 . J Biol Chem. 2010;285(20):15599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer MB, Pike JW. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J Steroid Biochem Mol Biol. 2020;196:105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zehnder D, Bland R, Walker EA, et al. Expression of 25‐hydroxyvitamin D3‐1alpha‐hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10(12):2465–73. [DOI] [PubMed] [Google Scholar]
- 28. Zehnder D, Bland R, Williams M, et al. Extrarenal expression of 25‐hydroxyvitamin d(3)‐1 alpha‐hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–94. [DOI] [PubMed] [Google Scholar]
- 29. Bland R, Zehnder D, Hewison M. Expression of 25‐hydroxyvitamin D3‐1alpha‐hydroxylase along the nephron: new insights into renal vitamin D metabolism. Curr Opin Nephrol Hypertens. 2000;9(1):17–22. [DOI] [PubMed] [Google Scholar]
- 30. Bland R, Zehnder D, Hughes SV, Ronco PM, Stewart PM, Hewison M. Regulation of vitamin D‐1alpha‐hydroxylase in a human cortical collecting duct cell line. Kidney Int. 2001;60(4):1277–86. [DOI] [PubMed] [Google Scholar]
- 31. Kawashima H, Torikai S, Kurokawa K. Localization of 25‐hydroxyvitamin D3 1 alpha‐hydroxylase and 24‐hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981;78(2):1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cusanovich DA, Hill AJ, Aghamirzaie D, Daza RM, Pliner HA, Berletch JB, et al. A single‐cell atlas of in vivo mammalian chromatin accessibility. Cell. 2018;174(5):1309‐1324.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA‐binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC‐seq: a method for assaying chromatin accessibility genome‐wide. Curr Protoc Mol Biol. 2015;109:21.9.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buenrostro JD, Wu B, Litzenburger UM, et al. Single‐cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523(7561):486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hewison M, Burke F, Evans KN, et al. Extra‐renal 25‐hydroxyvitamin D3‐1alpha‐hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–21. [DOI] [PubMed] [Google Scholar]
- 37. Adams JS, Hewison M. Extrarenal expression of the 25‐hydroxyvitamin D‐1‐hydroxylase. Arch Biochem Biophys. 2012;523(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams JS, Rafison B, Witzel S, et al. Regulation of the extrarenal CYP27B1‐hydroxylase. J Steroid Biochem Mol Biol. 2014;144:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naja RP, Dardenne O, Arabian A, St Arnaud R. Chondrocyte‐specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25‐dihydroxyvitamin D3 in growth plate development. Endocrinology. 2009;150(9):4024–32. [DOI] [PubMed] [Google Scholar]
- 40. Adams JS, Singer FR, Gacad MA, et al. Isolation and structural identification of 1,25‐dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 1985;60(5):960–6. [DOI] [PubMed] [Google Scholar]
- 41. Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25‐dihydroxyvitamin D. N Engl J Med. 1981;305(8):440–3. [DOI] [PubMed] [Google Scholar]


