Abstract
Background
KRAS is mutated in ∼90% of pancreatic ductal adenocarcinomas, ∼35% of colorectal cancers and ∼20% of non-small-cell lung cancers. There has been recent progress in targeting G12CKRAS specifically, but therapeutic options for other mutant forms of KRAS are limited, largely because the complexity of downstream signaling and feedback mechanisms mean that targeting individual pathway components is ineffective.
Design
The protein kinases RAF and SRC are validated therapeutic targets in KRAS-mutant pancreatic ductal adenocarcinomas, colorectal cancers and non-small-cell lung cancers and we show that both must be inhibited to block growth of these cancers. We describe CCT3833, a new drug that inhibits both RAF and SRC, which may be effective in KRAS-mutant cancers.
Results
We show that CCT3833 inhibits RAF and SRC in KRAS-mutant tumors in vitro and in vivo, and that it inhibits tumor growth at well-tolerated doses in mice. CCT3833 has been evaluated in a phase I clinical trial (NCT02437227) and we report here that it significantly prolongs progression-free survival of a patient with a G12VKRAS spindle cell sarcoma who did not respond to a multikinase inhibitor and therefore had limited treatment options.
Conclusions
New drug CCT3833 elicits significant preclinical therapeutic efficacy in KRAS-mutant colorectal, lung and pancreatic tumor xenografts, demonstrating a treatment option for several areas of unmet clinical need. Based on these preclinical data and the phase I clinical unconfirmed response in a patient with KRAS-mutant spindle cell sarcoma, CCT3833 requires further evaluation in patients with other KRAS-mutant cancers.
Key words: KRAS, panRAF/SRC inhibitor, CRC, PDAC, NSCLC
Highlights
-
•
We synthesized new drug, CCT3833, that inhibits both RAF and SRC, and so may be effective in KRAS-mutant cancers.
-
•
CCT3833 inhibits both signaling pathways in vitro and in vivo, inhibits tumor growth and leads to regressions in mice.
-
•
CCT3833 significantly prolonged progression-free survival of a patient with a G12VKRAS spindle cell sarcoma.
-
•
CCT3833 is a potential treatment option for several areas of unmet clinical need.
Introduction
Lung cancer [∼90% of which are non-small-cell lung cancers (NSCLC)] is the most common cancer worldwide and in the UK has a 5-year overall survival (OS) of only 15%.1,2 Colorectal cancer (CRC) is the third most common cancer1 and in the UK has a 5-year OS of 60%2, and pancreatic ductal adenocarcinoma (PDAC) is the tenth most common cancer in the UK and has the poorest prognosis with 5-year OS of only 5%.2 These poor survival rates are partly due to a lack of treatment options. Surgery is the preferable treatment of NSCLC, PDAC and CRC, but most patients present late with inoperable advanced disease and so receive systemic therapy.3,4 Targeted therapies are licensed for NSCLC (EGFR, ALK, ROS1 indications) and CRC (KRAS wild-type), but KRAS-mutated cancer remains an area of unmet clinical need. Critically, KRAS is mutated in ∼20% NSCLC, ∼90% PDAC and ∼35% CRC.5 These patients receive conventional chemotherapy or immunotherapy, often with limited efficacy and potential toxicity4,6,7 except for KRAS-mutant NSCLC patients who benefit from immune check-point inhibitors compared with KRAS wild-type patients.8,9
Thus, although RAS (KRAS, NRAS, HRAS) is mutated in ∼25% of all cancers, treatment of these patients is challenging.10 Notably, direct inhibitors of KRAS are limited to the p.G12C KRAS-mutant,11, 12, 13 so an alternative is to target downstream effectors in the RAF/MEK/ERK pathway, which has led to the development of RAF, MEK and ERK drugs.14 However, in KRAS-mutant cells, BRAF-selective drugs such as vemurafenib (PLX4032) and dabrafenib15 cause paradoxical hyperactivation of the RAF-ERK pathway through formation of BRAF-CRAF homo- and hetero-dimers.16 Unfortunately, targeting MEK downstream of RAF with drugs such as trametinib17 is ineffective in KRAS-mutant cancers because of feedback mechanisms18 and adverse side-effects,19 and therefore these drugs have been unsuccessful in KRAS-mutant PDAC, CRC and NSCLC.20,21
With a pressing need for different approaches for KRAS-mutant cancers, here we describe a new drug for this indication. The protein kinase SRC is a master regulator of cancer cell proliferation, metastasis and invasion, and it also potentiates cancer processes such as neo-angiogenesis.22 SRC-family kinases (SFKs) are associated with pathogenesis of many cancers, particularly late-stage disease, where its increased activity and expression are associated with disease progression and poorer prognosis. Critically, SRC is a validated target in KRAS-mutant CRC,23 PDAC22,24 and NSCLC22,25 and it is known that G12CKRAS and SRC inhibitors work synergistically to inhibit G12CKRAS NSCLC cell proliferation.26
In this study, we describe CCT3833, a new combined panRAF and SRC inhibitor. We show that CCT3833 does not drive paradoxical activation of the RAF/MEK/ERK pathway in KRAS-mutant cells and that its ability to exert dual inhibition of RAF and SRC provides effective therapy in preclinical KRAS-mutant PDAC, CRC and NSCLC models. We show that CCT3833 is superior to single-agent panRAF or SRC inhibitors and comparable with combination panRAF + SRC inhibitors in standard two-dimensional tissue culture and more significantly, in three-dimensional spheroids, which are of intermediate complexity between standard monolayer cultures in vitro and tumors in vivo.
Importantly, CCT3833 has been investigated in a phase I dose-escalation clinical trial (NCT02437227) including 31 patients with solid tumors, of whom at least 10 were known to be KRAS-mutant. We report an unconfirmed partial response and prolonged clinical benefit from CCT3833 in one of these patients, diagnosed with a KRAS-mutant spindle cell sarcoma. This was the only patient with an unconfirmed partial response on trial. Spindle cell sarcomas are connective tissue tumors characterized by spindle-shaped cells, and are typically treated with anthracyclines, but with limited and variable responses.27 Here, we describe a patient with a spindle cell sarcoma presenting a p.G12V KRAS mutation. The patient displayed early disease progression following surgical resection, was not a candidate for doxorubicin chemotherapy and did not respond to the multikinase inhibitor pazopanib. Despite being in the dose escalation phase, CCT3833 achieved a progression-free survival (PFS) of >10 months, and we provide comprehensive analysis of the mechanism of action of CCT3833 in KRAS-mutant cancers to reveal how this patient and others could benefit from this agent.
Materials and methods
Cell culture
Cell lines were cultured under standard conditions. Human PDAC cell lines (except for MIA-PaCa2) were a gift from Dr Claus Jorgensen, Calu-1 and H460 cells were a gift from Dr Michela Garofalo and H2009 NSCLC cells were a gift from Dr John Brognard. All other human cell lines were from the American Type Culture Collection (ATCC). Short tandem repeat profiles were routinely compared with known ATCC fingerprints and cells were routinely ensured to be mycoplasma free by PCR. Mouse KPC PDAC cells were a gift of Professor Owen Sansom, or were isolated and established in house from transgenic mice as described.24 Cells were cultured in Dulbecco's modified Eagle's Medium or RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Short-term growth inhibition assays, long-term cell proliferation assays and tumor spheroid assays were carried out as detailed in Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2020.10.483. A list of the cell lines used and their KRAS status is detailed in Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2020.10.483.
Mouse allograft/xenograft studies
All animal procedures were carried out in accordance with National Home Office regulations under the Animals (Scientific Procedures) Act 1986 under license PPL-70/7635 and PPL-70/7701 and within guidelines set out by the CRUK Manchester Institute and The Institute of Cancer Research Animal Welfare and Ethical Review Bodies, and described in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. In vivo efficacy and pharmacodynamics studies were carried out as detailed in Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2020.10.483.
Statistics
For graphs, mean values are shown and error bars represent standard error of the mean unless stated otherwise, (∗) P ≤ 0.05 (Student's or Welch's t-test as indicated). All in vitro experiments were carried out in triplicate unless otherwise stated.
Results
CCT3833 is a panRAF plus SFK inhibitor
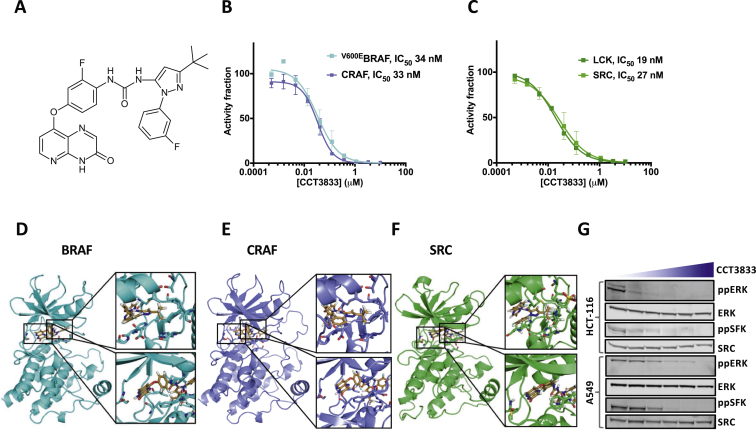
CCT3833, (1-[3-tert-butyl-1-[(3-fluoro-phenyl)-1H-pyrazol-5-yl]3-[2-fluoro-4(3-oxo-3,4-dihydropyrido[2,3-b]pyrazin-8-yloxy)phenyl]urea; Figure 1A) is a panRAF inhibitor that inhibits V600EBRAF at 34 nM and CRAF at 33 nM (Figure 1B, Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2020.10.483). In selectivity screens CCT3833 is mostly inactive against other kinases, with the important exception of SFKs (Supplementary Figure S1A, available at https://doi.org/10.1016/j.annonc.2020.10.483) with SRC inhibition at 27 nM inhibition of the SFK lymphocyte-specific protein tyrosine kinase at 19 nM (Figure 1C, Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2020.10.483).
Figure 1.
CCT3833 is a panRAF/SRC inhibitor active in KRAS-mutant cells.
(A) CCT3833 chemical structure. (B) In vitro enzyme assay for V600EBRAF or CRAF and (C) SRC or LCK incubated with increasing concentrations of CCT3833. (D) CCT3833 docked into BRAF binding site (pdb4JVG). Inset, detailed CCT3833 interactions with allosteric site (top), ATP binding site (bottom) of BRAF (turquoise). (E) CCT3833 docked into CRAF binding site (homology model derived from pdb4JVG) (orchid). Inset, CCT3833 interactions with allosteric site (top), ATP binding site (bottom). (F) CCT3833 docked into SRC binding site (pdb4AGW) (green). Inset, interactions of CCT3833 with allosteric site (top) and ATP binding site (bottom). (G) Immunoblot for ppERK/ERK and ppSFK/SRC in HCT-116 and A549 cells after 4 h with dimethyl sulfoxide (first lane) or CCT3833 (0.6, 1.2, 2.5, 5, 10 μM respectively). LCK, lymphocyte-specific protein tyrosine kinase; SFK, SRC-family kinase.
Docking studies predict that CCT3833 is a type II inhibitor that binds to the inactive ‘DFG-out’ conformation of BRAF,28 and to CRAF and SRC through similar mechanisms (Figure 1D-F). Specifically, the pyridopyrazinone moiety is predicted to interact with the kinase hinge, the central aromatic ring occupies the ATP binding pocket and the pyrazole ring elaborates into an allosteric site created by the DFG moving into the ‘out’ conformation (Figure 1D-F). Moreover, the tert-butyl group is predicted to elaborate into a hydrophobic pocket and the terminal fluoro-substituted phenyl ring points towards the activation loop in all three kinases (Figure 1D-F). These binding similarities indicate how CCT3833 inhibits both RAF and SRC and we confirm that CCT3833 inhibits both ERK (ppERK; downstream of CRAF) and SFK (ppSFK) phosphorylation in a dose-dependent manner in HCT-116 (CRC), A549 (NSCLC) (Figure 1G) and MIA-PaCa2 (PDAC) cells (Supplementary Figure S1B, available at https://doi.org/10.1016/j.annonc.2020.10.483). Our docking studies predict a steric clash would occur between the pyridopyrazinone moiety of CCT3833 and a T338I (the so-called gatekeeper residue) substitution in chicken SRC (Supplementary Figure S2A, available at https://doi.org/10.1016/j.annonc.2020.10.483) and accordingly, we show that CCT3833 inhibits wild-type SRC but not T341ISRC (the human equivalent of T338I) in either HEK-293 or HCT-116 cells (Supplementary Figure S2B-E, available at https://doi.org/10.1016/j.annonc.2020.10.483), supporting our predictions for binding mechanism.
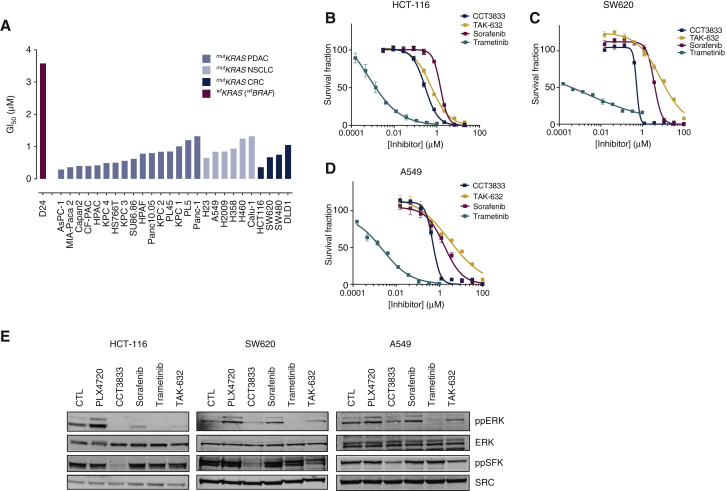
CCT3833 inhibits KRAS-mutant cancer cell growth
The data above show that CCT3833 is a panRAF inhibitor that also inhibits SRC. Notably, RAF and SRC are validated targets in RAS-mutant cancers, because RAF signals downstream of oncogenic KRAS, and SFKs drive cancer cell proliferation and survival. Accordingly, we show that CCT3833 is active against a panel of KRAS-mutant PDAC, CRC and NSCLC cell lines, whereas it is less potent against KRAS/BRAF wild-type cells (Figure 2A, Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2020.10.483). Moreover, compared with other RAF inhibitors, in short-term growth assays CCT3833 inhibits HCT-116 growth more potently than the clinically evaluated panRAF inhibitors TAK-632, ARQ736 and MLN-2480 (Figure 2B, Supplementary Figure S3A, available at https://doi.org/10.1016/j.annonc.2020.10.483). We also show that CCT3833 is more effective than the multikinase inhibitor sorafenib or the BRAF-mutant selective inhibitors PLX4720 and dabrafenib, and that only the MEK inhibitor trametinib is more potent than CCT3833 at inhibiting HCT-116 cells (Figure 2B, Supplementary Figure S3A, available at https://doi.org/10.1016/j.annonc.2020.10.483). We observe similar responses in SW620 (CRC; Figure 2C, Supplementary Figure S3B, available at https://doi.org/10.1016/j.annonc.2020.10.483), A549 (Figure 2D), MIA-PaCa2 (Supplementary Figure S3C and D, available at https://doi.org/10.1016/j.annonc.2020.10.483) and Calu-1 cells (NSCLC, Supplementary Figure S3E, available at https://doi.org/10.1016/j.annonc.2020.10.483), where CCT3833 inhibits growth more effectively than all other RAF inhibitors, with only trametinib being significantly more potent.
Figure 2.
CCT3833 is active in KRAS-mutant cells via on target modulation.
(A) CCT3833 GI50s in KRAS-mutant and D24 WTKRAS cell lines. (B-D) Proliferation of HCT-116 (B), SW620 (C), A549 (D) cells treated with increasing concentrations of the indicated drugs. (E) Immunoblot for ppERK/ERK and ppSFK/SRC in HCT-116, SW620 and A549 cells after 4 h with dimethyl sulfoxide (DMSO) (CTL), PLX4720, CCT3833, sorafenib, TAK-632 (all at 1 μM), or trametinib (20 nM). SFK, SRC-family kinase.
Although the panRAF inhibitor TAK-632, and the BRAF inhibitors PLX4720 and dabrafenib inhibit BRAF more potently than CCT3833 in in vitro enzyme assays, CCT3833 is more potent at inhibiting KRAS-mutant cancer cell growth, so we examine downstream signaling. In HCT-116, SW620, A549, MIA-PaCa2 and Calu-1 cells, PLX4720 induces paradoxical activation of the ERK pathway and although sorafenib and TAK-632 inhibit ppERK, they are less potent than CCT3833 in their ability to do so (Figure 2E, Supplementary Figure S3F-G, available at https://doi.org/10.1016/j.annonc.2020.10.483). Note also that PLX4720, sorafenib and TAK-632 do not inhibit ppSFK in these cells, whereas CCT3833 potently inhibits ppSFK (Figure 2E, Supplementary Figure S3F-G, available at https://doi.org/10.1016/j.annonc.2020.10.483). Finally, although trametinib inhibits ppERK more effectively than CCT3833 in these cells, unlike CCT3833 it fails to inhibit ppSFK (Figure 2E, Supplementary Figure S3F-G, available at https://doi.org/10.1016/j.annonc.2020.10.483).
RAF and SFK must both be inhibited to block KRAS-mutant cancer growth
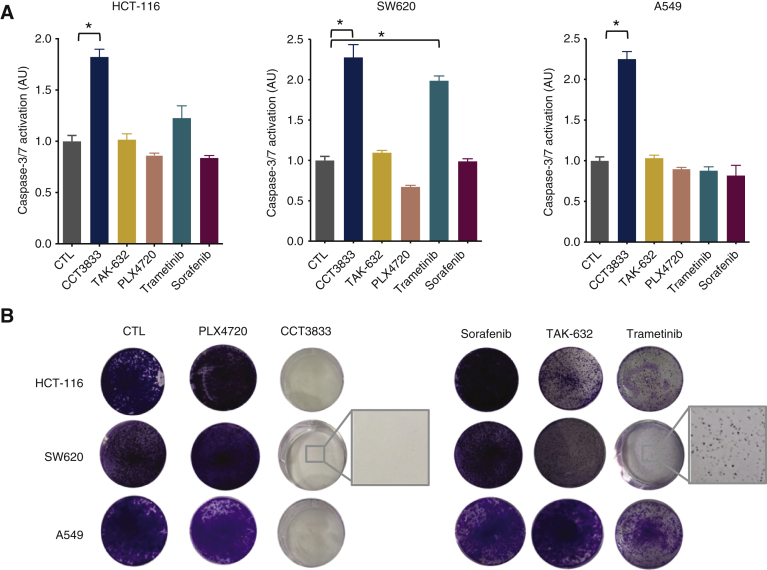
Thus, CCT3833 inhibits both CRAF and SRC in KRAS-mutant cancers and so we investigate the contribution of these two activities to the inhibition of long-term cell growth. We show that CCT3833 induces significant caspase-3/7 activation, whereas PLX4720, sorafenib, trametinib and TAK-632 do not activate caspase-3/7 to the same extent (Figure 3A, Supplementary Figure S3H, available at https://doi.org/10.1016/j.annonc.2020.10.483). Accordingly, in long-term clonogenic proliferation assays, only CCT3833 fully inhibits HCT-116, SW620, A549 and MIA-PaCa2 cell growth, whereas colonies are still evident with PLX4720, sorafenib, TAK-632 and also trametinib (Figure 3B, Supplementary Figure S3I and J, available at https://doi.org/10.1016/j.annonc.2020.10.483). These findings are confirmed in two additional human PDAC cell lines (Supplementary Figure S4, available at https://doi.org/10.1016/j.annonc.2020.10.483). Note also that the inhibitors are used at doses to reflect safe plasma exposure achievable in vivo. Thus, trametinib is used at 20-30 nM, the maximum tolerated patient plasma concentration,17,29 whereas CCT3833 is used at 1 μM, below the well-tolerated mouse plasma concentration in vivo (vide infra).
Figure 3.
CCT3833 inhibits cell growth and induces apoptosis.
(A) Caspase-3/7 activation in HCT-116, SW620 and A549 cells after dimethyl sulfoxide (DMSO) (CTL), CCT3833, TAK-632, PLX4720, sorafenib (all at 1 μM), or trametinib (20 nM), ∗P ≤ 0.05 Student's t-test. (B) Long-term proliferation assay on HCT-116, SW620 and A549 cells after 9-12 days of treatment with DMSO (CTL), PLX4720, CCT3833, sorafenib, TAK-632 (all at 1 μM), or trametinib (20 nM). Insets are high magnification images of area indicated by squares.
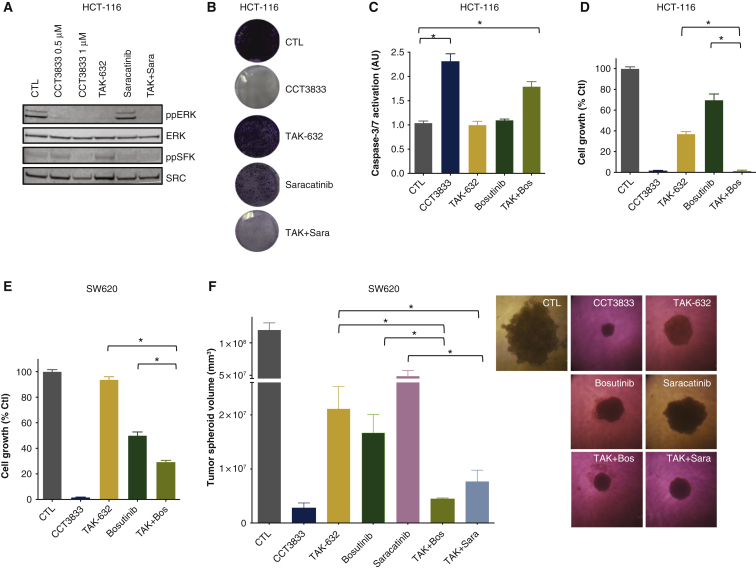
To assess whether it is necessary to inhibit both ERK and SRC pathways in KRAS-mutant cells, we combine known panRAF and SRC inhibitors. As shown earlier, TAK-632 inhibits ppERK but not ppSFK and conversely, we show that the SRC inhibitor saracatinib inhibits ppSFK but not ppERK (Figure 4A). Moreover, together these agents mimic the effects of CCT3833 (Figure 2E) and inhibit both ppERK and ppSFK (Figure 4A). In long-term clonogenic growth assays, neither TAK-632 nor saracatinib alone inhibit colony formation, whereas together they do inhibit colony formation, both in HCT-116 cells and in H23 lung adenocarcinoma cells, again mimicking the effects of CCT3833 alone (Figure 4B, Supplementary Figure S5A, available at https://doi.org/10.1016/j.annonc.2020.10.483). We also assess another SRC inhibitor, bosutinib. Alone, bosutinib does not activate caspase-3/7, but it co-operates with TAK-632 to activate these pro-apoptosis enzymes (Figure 4C) mimicking the effect of CCT3833 alone. Moreover, TAK-632 and bosutinib co-operate to inhibit the short-term growth of HCT-116, SW620 (Figure 4D-E), A549 and MIA-PaCa2 cells (Supplementary Figure S5B, available at https://doi.org/10.1016/j.annonc.2020.10.483), again mimicking the effect of CCT3833 alone. Notably, the combinations of saracatinib plus TAK-632 or bosutinib plus TAK-632 both inhibit the growth of SW620 tumor spheroids similarly to CCT3833 alone, whereas the response to the single agents is significantly less (Figure 4F).
Figure 4.
RAF and SRC dual inhibition is required to efficiently inhibit KRAS-mutant cell growth.
(A) Immunoblot for ppERK/ERK and ppSFK/SRC in HCT-116 cells, 24 h with dimethyl sulfoxide (DMSO) (CTL), CCT3833 (0.5 μM, 1 μM), TAK-632 and saracatinib (both at 1 μM) or TAK-632 plus saracatinib (TAK + Sara; 1 μM each). (B) Long-term proliferation on HCT-116 cells, 10 days, DMSO (CTL), CCT3833, TAK-632, saracatinib (all at 1 μM) or TAK-632 plus saracatinib (TAK + Sara; 1 μM each). (C) Caspase-3/7 activation in HCT-116 cells with DMSO (CTL), CCT3833 (1.2 μM), TAK-632 (1.2 μM), bosutinib (2 μM) or TAK-632 plus bosutinib (TAK + Bos; 1.2 + 2 μM), ∗P ≤ 0.05 Student's t-test. (D, E) Short-term cell proliferation assays of HCT-116 (D) and SW620 (E) cells treated with CCT3833 (1.2 μM), TAK-632 (1.2 μM), bosutinib (2 μM) or TAK-632 plus bosutinib (TAK + Bos; 1.2 + 2 μM, ∗P ≤ 0.05 Student's t-test. (F) SW620 spheroids treated with DMSO (CTL), CCT3833, TAK-632 and saracatinib (all 5 μM), bosutinib (1.2 μM) or TAK-632 plus bosutinib (TAK + Box; 5 μM + 1.2 μM) or TAK-632 plus saracatinib (TAK + Sara; 5 μM each) for 5 days. Volume = [(width2 × length)/2], data shown as mean +/− standard deviation, ∗P ≤ 0.05 Student's t-test. Representative images are shown on the right. SFK, SRC-family kinase.
CCT3833 inhibits KRAS-mutant tumor growth
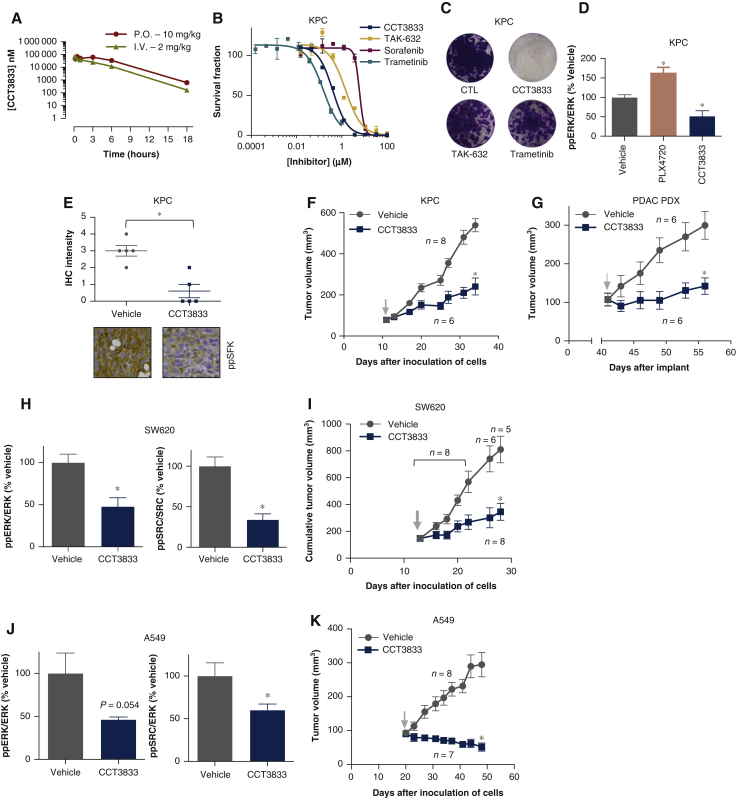
Next, we assess CCT3833 in vivo. We show that CCT3833 has good oral bioavailability in mice, excellent pharmacokinetic properties (Figure 5A, Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2020.10.483), achieves plasma and tumor concentrations well above the GI50 for the target cancer cells and does not accumulate following daily oral doses (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2020.10.483, Figure 2A).
Figure 5.
In vivo efficacy of CCT3833 in PDAC, CRC and NSCLC.
(A) CCT3833 pharmacokinetics studies carried out in BALB/c mice: plasma concentrations at timepoint 18 h show concentration of ∼1 μM when administered by oral gavage. P.O. = oral administration (10 mg/kg); I.V. = intravenous administration (2 mg/kg) in 5% dimethyl sulfoxide (DMSO), 95% water. (B) Proliferation for KPC mouse cells with different drugs. (C) Long-term proliferation of KPC cells, 9 days with DMSO (CTL), CCT3833, TAK-632 (all at 1 μM), trametinib (20 nM). (D) Immunoblot quantification of ppERK/ERK in tumors from the biomarker study in KPC allografts, by oral gavage (p.o.) 4 days with vehicle control (5% DMSO/water), PLX4720 90 mg/kg or CCT3833 (40 mg/kg). See Supplementary Figure S7A, available at https://doi.org/10.1016/j.annonc.2020.10.483, for the blots, ∗P ≤ 0.05 Student's t-test. (E) Immunohistochemistry and scoring of the ppSFK intensity in tumors from the biomarker study in KPC allografts, ∗P ≤ 0.05 Student's t-test. Representative images are shown below. (F) Tumor growth curves for KPC allografts, with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), p.o., qd, 23 days, ∗P ≤ 0.05 Welch's t-test on day 23 of treatment. (G) Tumor growth curves for a PDAC PDX, with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), p.o., qd, 15 days, ∗P ≤ 0.05 Welch's t-test on day 15 of treatment. (H) Immunoblot quantification of ppERK/ERK, ppSFK/SRC in SW620 xenografts from biomarker study, p.o., for 4 days with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), ∗P ≤ 0.05 Student's t-test. See Supplementary Figure S7C, available at https://doi.org/10.1016/j.annonc.2020.10.483, for blots. (I) Cumulative tumor growth curves for SW620 xenografts, with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), p.o., qd, 15 days, ∗P ≤ 0.05 Welch's t-test on day 15 of treatment. (J) Immunoblot quantification of ppERK/ERK, ppSFK/ERK in A549 xenografts from biomarker study, p.o., for 4 days, with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), ∗P ≤ 0.05 Student's t-test. See Supplementary Figure S7E, available at https://doi.org/10.1016/j.annonc.2020.10.483, for blots. (K) Tumor growth curves for A549 xenografts, with vehicle control (5% DMSO/water) or CCT3833 (40 mg/kg), p.o., qd, 28 days, ∗P ≤ 0.05 Welch's t-test on day 28 of treatment. ↓ indicates start of treatment of all tumor growth curves. PDAC, pancreatic ductal adenocarcinoma; PDX, patient-derived xenograft; SFK, SRC-family kinase.
We tested CCT3833 in a mouse model of PDAC driven by oncogenic KRAS and inactivating mutation of the tumor suppressor TP53 (KPC cells24). We confirm, commensurate with our human cell observations, that, CCT3833 is more effective than the other pathway inhibitors apart from trametinib at blocking KPC cell growth in short-term proliferation assays, but only CCT3833 completely abrogates growth of these cells in long-term assays (Figure 5B and C, Supplementary Figure S6A and B, available at https://doi.org/10.1016/j.annonc.2020.10.483). Accordingly, CCT3833 is more potent at inducing caspase-3/7 activation (Supplementary Figure S6C and D, available at https://doi.org/10.1016/j.annonc.2020.10.483). We show CCT3833 blocks ERK and SFK phosphorylation and suppresses tumor growth in KPC cells grown as allografts in mice (Figure 5D-F, Supplementary Figure S7A, available at https://doi.org/10.1016/j.annonc.2020.10.483). Critically, CCT3833 inhibits a human KRAS-mutant PDAC patient-derived xenograft (PDX)(Figure 5G, Supplementary Figure S7B, available at https://doi.org/10.1016/j.annonc.2020.10.483).
Importantly, we show that CCT3833 is effective in other human KRAS-mutant cells in vivo. It inhibits ppERK and ppSFK in SW620 xenografts and suppresses the growth of these CRC tumors in immunocompromised mice (Figure 5H and I, Supplementary Figure S7C and D, available at https://doi.org/10.1016/j.annonc.2020.10.483). Finally, CCT3833 also inhibits ppERK and ppSFK in lung A549 cells and more importantly, at doses that are well tolerated in mice (Supplementary Figure S8, available at https://doi.org/10.1016/j.annonc.2020.10.483), CCT3833 inhibits ERK and SRC, and causes regression of A549 tumors xenografts in mice (Figure 5J and K), and it mediates a significant reduction in the size of foci in the A549 pseudo metastasis tail vein injection model (Supplementary Figure S7E-I, available at https://doi.org/10.1016/j.annonc.2020.10.483).
CCT3833 improves progression-free survival in a patient with G12VKRAS spindle cell sarcoma
A previously fit patient aged in their 70s presented with a 1 year history of non-specific symptoms and was diagnosed with a large intra-abdominal mass associated with the pancreas and invading into the liver parenchyma (Figure 6A). This was resected and histopathological assessment revealed a lobulated tumor composed of ill-defined fascicles of spindle cells, with oval to elongated moderately pleomorphic nuclei, pale eosinophilic cytoplasm and up to 9 mitoses per 10 high powered fields, later classified as a spindle cell sarcoma NOS (not otherwise specified). Immunohistochemistry was diffusely positive for CD34, but negative for other markers including S-100, SOX10, DOG1, CD117, SMA, desmin, AE1-3, EMA, CD21 and CD23. Moreover, the tumor was negative for the NAB2-STAT6 fusion transcripts.
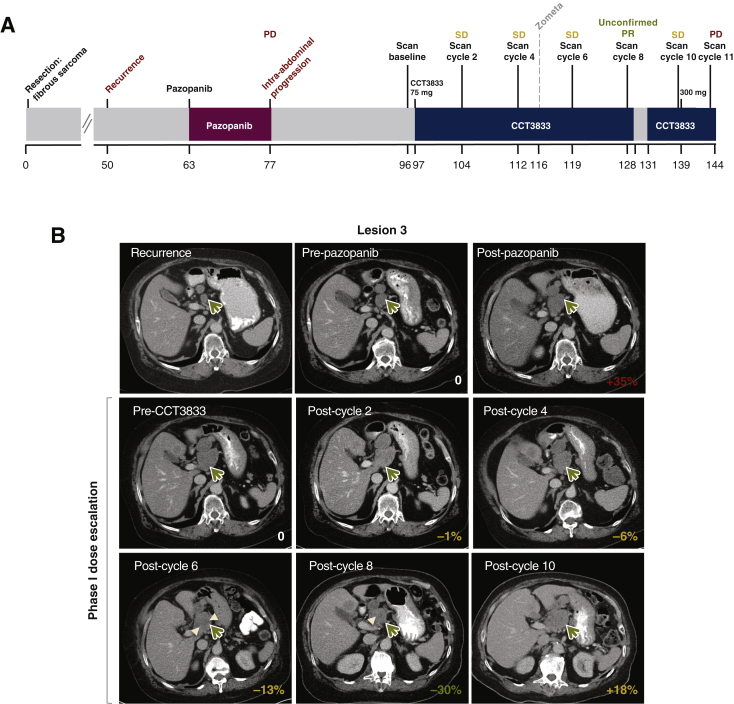
Figure 6.
Clinical response in a patient with KRAS-mutant spindle cell sarcoma NOS.
(A) Patient clinical history. (B) Computed tomogrpahy scans of lesion 3 (mass superior to pancreatic head, green arrow, see also Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2020.10.483, for blots) of the patient at recurrence, before and after pazopanib treatment; before and during CCT3833 treatment. 0 = baseline (pretreatment) before each treatment; percentage of sum of all marker lesions is reported on the bottom right of each scan as per RECIST 1.1 criteria (Table 1). Hypodensity within the lesion seen on scan at cycle 6 and 8 suggests necrosis due to treatment response (white arrowhead). NOS, not otherwise specified; PD, progressive disease; unconfirmed PR, unconfirmed partial response; SD, stable disease.
One year after tumor resection, the patient presented with a multifocal intra-abdominal recurrence, which was inoperable. Although it was later diagnosed as spindle cell sarcoma, due to some histopathological features, it was treated as a malignant solitary fibrous tumor. The patient commenced on the multikinase inhibitor pazopanib, but had extensive disease progression at first radiological assessment after only 12 weeks of treatment (Figure 6A and B, Table 1, Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2020.10.483). The patient was not a candidate for doxorubicin chemotherapy and molecular testing was carried out using a 19-gene MassArray assay (Sequenom Oncocarta v1.0, San Diego, CA), which revealed a KRAS C35G>Tp.Gly12Val mutation, so the patient was enrolled on to the phase I trial of CCT3833 (NCT02437227) and was allocated to the lead-in dose CCT3833 (75 mg once a day, continuous dosing). Scans were taken at baseline and every 8 weeks during CCT3833 treatment (Figure 6B). In stark contrast to the progression seen with pazopanib, each of the scans after commencing CCT3833 treatment show stable disease with achievement of an unconfirmed partial response after eight cycles as defined by RECIST 1.1 (Figure 6B, Table 1, Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2020.10.483). As a non-RECIST progression was seen on imaging after cycle 10, the patient underwent intrapatient dose escalation to 300 mg once daily, but a scan on cycle 11 (Table 1) confirmed RECIST disease progression and the patient discontinued treatment on day 17 of cycle 12.
Table 1.
Patient scan marker lesion measurements (RECIST version 1.1) at baseline and on treatment.
| Weeks from diagnosis | Drug | Cycle | Lesion1 | Lesion2 | Lesion3 | Lesion4 | Lesion5 | Total (cm) | % Change |
|---|---|---|---|---|---|---|---|---|---|
| 62.7 | – | 0 | 1.5 | 2.2 | 3.0 | 1.0 | 0.5 | 8.2a | 0 |
| 75.7 | Pazopanib | 3 | 2.2 | 2.5 | 4.0 | 1.5 | 0.9 | 11.1 | +35 |
| 95.9 | – | 0 | 3.3 | 3.1 | 4.7 | 2.1 | 1.0 | 14.2a | 0 |
| 104.3 | CCT3833 | 2 | 2.9 | 3.7 | 4.5 | 2.0 | 0.9 | 14.0 | −1 |
| 112.3 | CCT3833 | 4 | 2.9 | 3.4 | 4.2 | 2.3 | 0.5 | 13.3 | −6 |
| 118.6 | CCT3833 | 6 | 2.6 | 2.7 | 4.1 | 2.4 | 0.5 | 12.3 | −13 |
| 128.3 | CCT3833 | 8 | 1.7 | 2.2 | 3.6 | 2.0 | 0.5 | 10.0b | −30 |
| 138.6 | CCT3833 | 10 | 1.7 | 2.7 | 4.5 | 2.9 | 0.0 | 11.8 | +18 |
| 143.3 | CCT3833 | 11 | 1.8 | 3.0 | 4.8 | 3.2 | 0.0 | 12.8 | +28 |
Baseline measurements.
Nadir.
Discussion
Herein, we report that the new inhibitor, CCT3833, mediated an unconfirmed partial response in a patient with aggressive KRAS-mutant spindle cell sarcoma who was not eligible for surgery or chemotherapy, and who did not respond to the multikinase inhibitor pazopanib. Despite being in the dose-escalation phase of the clinical trial (NCT02437227), at 75 mg p.o. qd continuous dosing of CCT3833, the patient achieved progression-free survival for 8 months, and did not progress until the 12th cycle of treatment. Together with our preclinical data, this indicates that CCT3833 has potential for the treatment of KRAS-mutant tumors. Specifically, our preclinical data demonstrate that CCT3833 is effective in KRAS-mutant CRC, NSCLC and PDAC due to its dual anti-panRAF plus anti-SRC activity. RAF is a validated target directly downstream of oncogenic RAS, and SRC is also a validated therapeutic target in CRC, NSCLC and PDAC, where it is hyperactivated and drives cell proliferation and metastasis.22, 23, 24, 25 Accordingly, SRC inhibitors cooperate with drugs that target the EGFR/RAS pathway.26,30,31
Our findings that CCT3833 inhibits the growth of KRAS-driven murine PDAC in vitro, and in vivo, are aligned to the literature, validating SRC as a therapeutic target in PDAC, where its overexpression or hyperactivation are markers of poor clinical outcome.24 Moreover, SRC inhibitors are active in preclinical PDAC models and achieve minor clinical responses in PDAC patients.22 SRC is similarly overexpressed or hyperactivated in CRC23 and moreover, BRAF-mutant CRC cells can switch between RAF/MEK/ERK and receptor tyrosine kinase signaling,32 so cell growth is only prevented when both pathways are inhibited. This plasticity may explain the shorter duration of response to BRAF and MEK inhibitors in CRC21 and may also underpin why mutant KRAS opposes the antitumor effects of EGFR inhibitors in CRC.33 Notably, MEK and EGFR inhibitors cooperate to block EGFR inhibitor-resistant CRC tumor growth,33 and we propose therefore that CCT3833 is effective in CRC because it targets the two key pathways downstream from mutant RAS and the hyperactivated receptor tyrosine kinases such as EGFR. Similarly, synergistic efficacy has been shown in vivo by inhibiting the MAPK pathway plus SRC in KRAS/PIK3CA double-mutant CRC cells.34
Finally, SRC and ERK signaling are both critical for the growth of KRAS-mutant NSCLC.20,25 Clinical trials with trametinib in KRAS-mutant NSCLC patients, alone or in combination with chemotherapy, show that single agent MEK inhibitors achieve no improvement compared with chemotherapy and that toxicity limits their clinical use in combination.19,20 Again, we posit that CCT3833 is effective in NSCLC because of its ability to simultaneously inhibit SRC and ERK signaling. Critically, CCT3833 mediates tumor regression in G12SKRAS-mutant NSCLC xenografts, so it could be considered for treatment of KRAS-mutant NSCLC patients who fail chemotherapy and/or immunotherapy.
In summary, we describe the discovery of CCT3833, a new panRAF/SRC inhibitor, and show that it is effective in KRAS-mutant cancer models, because RAF and SRC are central nodes in KRAS-mutant cancers. CCT3833 differs from the RAF dimer inhibitor LY300912035 because it also inhibits SRC and is effective in PDAC. We posit that CCT3833 inhibits tumor growth in RAS-mutant models through on-target inhibition of BRAF and CRAF, and additional on-target inhibition of SRC. Critically, CCT3833 induces tumor cell death and elicits therapeutic efficacy at well-tolerated doses in mice, and it is evaluated in patients in a phase I clinical trial, achieving a proof-of-concept unconfirmed clinical response in a patient with aggressive KRAS-mutant spindle cell sarcoma who was not eligible for other treatments. Taken together, our data support the further clinical evaluation of CCT3833 in patients with KRAS-mutant cancers.
Acknowledgements
We thank Professor Owen Sansom, Dr Claus Jorgensen, Dr John Brognard and Dr Michela Garofalo for providing cell lines. We thank Garry Ashton and Professor Martin Cook for the histology work and the BRU services for their help. We also thank Dr Juan Valle and Mr Derek O'Reilly for providing us with the PDAC patient sample to generate the PDX model. We acknowledge support by National Institute for Health Research (NIHR) Manchester Biomedical Research Centre, NIHR Manchester Clinical Research Facility at the Christie Hospital, and Manchester Experimental Cancer Medicine Centre, Manchester, UK. We thank Dr Geoff Kitson and Dr Keith Purdy for helpful discussion and support.
Funding
This work was supported by the Cancer Research UK Manchester Institute [grant numbers A27412, A22902, C5759/A17098]; and the Wellcome Trust [grant numbers WT1005X, 100282/Z/12/Z]; and the Division of Cancer Therapeutics at the Institute of Cancer Research [grant number C309/A11566]. C. Springer: ∼£3 million, Cancer Research UK (two grants); ∼£6 million Wellcome Trust; salaries CRUK Manchester Institute and Institute of Cancer Research. RM: ∼£3 million, Cancer Research UK (two grants); ∼£2.5 million Wellcome Trust; salary CRUK Manchester Institute. The clinical trial was supported by the National Institute for Health Research Biomedical Centre Grant to the Institute of Cancer Research and the Royal Marsden Hospital NHS Foundation Trust and Experimental Cancer Medicine Centre grants to the Institute of Cancer Research/Royal Marsden Hospital and the Christie Hospital.
Disclosure
Employees/former employees of the Institute of Cancer Research could benefit financially from commercialized drug discovery programs. RM consults for Pfizer. All remaining authors have declared no conflicts of interest.
Contributor Information
R. Marais, Email: richard.marais@manchester.ac.uk.
C. Springer, Email: caroline.springer@manchester.ac.uk.
Supplementary data
References
- 1.https://gco.iarc.fr Available at: Accessed November 2020.
- 2.www.cancerresearchuk.org Available at: Accessed November 2020.
- 3.Garrido-Laguna I., Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 4.Gustavsson B., Carlsson G., Machover D. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan B.A., Hughes B.G. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torralvo J., Friedlaender A., Achard V., Addeo A. The activity of immune checkpoint inhibition in KRAS mutated non-small cell lung cancer: a single centre experience. Cancer Genomics Proteomics. 2019;16:577–582. doi: 10.21873/cgp.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang A.C., Herbst R.S. Frontline immunotherapy for NSCLC—the tale of the tail. Nat Rev Clin Oncol. 2020;17:73–74. doi: 10.1038/s41571-019-0317-y. [DOI] [PubMed] [Google Scholar]
- 10.Mullard A. Cracking KRAS. Nat Rev Drug Discov. 2019;18:887–891. doi: 10.1038/d41573-019-00195-5. [DOI] [PubMed] [Google Scholar]
- 11.Hallin J., Engstrom L.D., Hargis L. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canon J., Rex K., Saiki A.Y. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 13.Hata A.N., Shaw A.T. Resistance looms for KRAS(G12C) inhibitors. Nat Med. 2020;26:169–170. doi: 10.1038/s41591-020-0765-z. [DOI] [PubMed] [Google Scholar]
- 14.Uehling D.E., Harris P.A. Recent progress on MAP kinase pathway inhibitors. Bioorg Med Chem Lett. 2015;25:4047–4056. doi: 10.1016/j.bmcl.2015.07.093. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty K.T., Puzanov I., Kim K.B. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karoulia Z., Gavathiotis E., Poulikakos P.I. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17:676–691. doi: 10.1038/nrc.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante J.R., Fecher L.A., Falchook G.S. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 18.Turke A.B., Song Y., Costa C. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 2012;72:3228–3237. doi: 10.1158/0008-5472.CAN-11-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janne P.A., Shaw A.T., Pereira J.R. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 20.Blumenschein G.R., Jr., Smit E.F., Planchard D. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC) dagger. Ann Oncol. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopetz S., Grothey A., Yaeger R. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 22.Kim L.C., Song L., Haura E.B. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 23.Lieu C., Kopetz S. The SRC family of protein tyrosine kinases: a new and promising target for colorectal cancer therapy. Clin Colorectal Cancer. 2010;9:89–94. doi: 10.3816/CCC.2010.n.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton J.P., Karim S.A., Graham K. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139:292–303. doi: 10.1053/j.gastro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Giaccone G., Zucali P.A. Src as a potential therapeutic target in non-small-cell lung cancer. Ann Oncol. 2008;19:1219–1223. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 26.Lito P., Solomon M., Li L.S. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351(6273):604–608. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collini P., Sorensen P.H., Patel S. Sarcomas with spindle cell morphology. Semin Oncol. 2009;36:324–337. doi: 10.1053/j.seminoncol.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Zambon A., Menard D., Suijkerbuijk B.M. Novel hinge binder improves activity and pharmacokinetic properties of BRAF inhibitors. J Med Chem. 2010;53:5639–5655. doi: 10.1021/jm100383b. [DOI] [PubMed] [Google Scholar]
- 29.Falchook G.S., Lewis K.D., Infante J.R. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn E.F., Iida M., Myers R.A. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene. 2011;30:561–574. doi: 10.1038/onc.2010.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields D.J., Murphy E.A., Desgrosellier J.S. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene. 2011;30:2123–2134. doi: 10.1038/onc.2010.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prahallad A., Sun C., Huang S. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 33.Misale S., Arena S., Lamba S. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:224ra226. doi: 10.1126/scitranslmed.3007947. [DOI] [PubMed] [Google Scholar]
- 34.Anderson G.R., Winter P.S., Lin K.H. A landscape of therapeutic cooperativity in KRAS mutant cancers reveals principles for controlling tumor evolution. Cell Rep. 2017;20:999–1015. doi: 10.1016/j.celrep.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng S.B., Henry J.R., Kaufman M.D. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell. 2015;28:384–398. doi: 10.1016/j.ccell.2015.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.