Abstract
As COVID vaccines roll out, internists and other health care providers are being turned to as trusted sources of information for patients and communities. Here, experts from NIAID outline the current state of knowledge regarding such vaccines. They contrast vaccine platforms, summarize clinical trial data regarding efficacy and safety, and comment on key questions including the ability of current vaccines to protect against infection and to decrease the prevalence of virus in the community.
Over the next weeks and months, physicians will face questions regarding the science, safety, and efficacy of the first wave of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccines to be authorized and distributed. In most cases these vaccine platforms will be new technologies that have not previously been administered other than through clinical trials. Although the initial data on efficacy and safety are extraordinarily encouraging, many questions remain regarding who should receive these vaccines and the immediate, intermediate, and long-term impact of the vaccination program on the pandemic. In this article, we provide a perspective on the vaccines furthest along in development in the United States, 2 of which have received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) and have been recommended for use by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). It is important to note that an EUA by the FDA is a mechanism used during a declared public health emergency to get potentially effective interventions as quickly as possible to those who might benefit and is not the same as formal FDA approval.
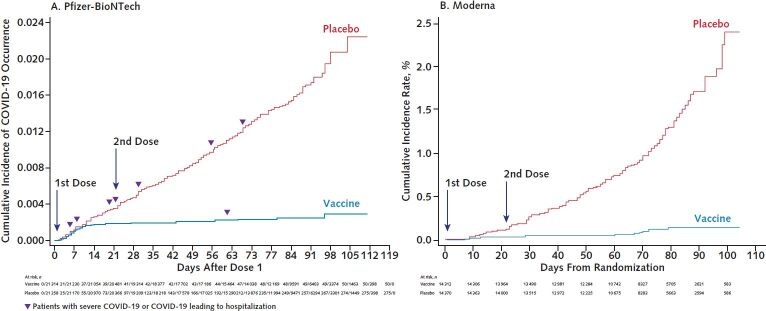
The vaccines developed by Pfizer-BioNTech and Moderna are the first coronavirus disease 2019 (COVID-19) vaccines to receive EUA and are currently being distributed according to the priorities set by the CDC (Table 1). These vaccines, and all vaccines currently under study in phase 2 or 3 trials in the United States, utilize the SARS-CoV-2 spike protein as their antigen. This glycoprotein is present on the surface of the SARS-CoV-2 virion, enables entry of the virion into cells through binding to angiotensin-converting enzyme II (ACE2), and is the primary target of neutralizing antibodies. The Pfizer-BioNTech and Moderna vaccines consist of synthetically produced messenger RNAs (mRNAs) that encode a stabilized form of the spike protein formulated in a lipid nanoparticle. Although the mRNA–lipid nanoparticle platform is not part of any currently licensed vaccine, it has been studied in humans for the past 10 years as investigational vaccine candidates for influenza virus, Ebola virus, and other diseases. Vaccines are deemed efficacious by preventing infection, transmission, mild disease, and/or severe disease. An interim analysis of the 2-dose regimen of the Pfizer-BioNTech COVID-19 vaccine observed 95% protection against symptomatic disease (1, 2); similar data were seen for the Moderna vaccine (3, 4) (Table 2). Although the total number of cases of severe disease were small in these trials, substantially fewer severe cases were observed in vaccine recipients than placebo recipients (Table 2). Of note, there was an indication of effectiveness even before the second dose for both the Pfizer-BioNTech and Moderna vaccines (Figure). Reactogenicity consistent with a vigorous immune response occurred in over half of the vaccine recipients, as evidenced by local injection-site reactions and mild systemic symptoms, such as myalgia and fatigue. However, the incidence of severe adverse events was balanced between the vaccine and placebo groups. The incidence and severity of local and systemic reactions to these vaccines were above those of seasonal influenza virus vaccines, and this should be made known to potential recipients before vaccination. However, as noted by the FDA Vaccines and Related Biological Products Advisory Committee, the risk–benefit analysis strongly favors immunization being safer for most individuals compared with the risks for infection.
Table 1. CDC Priorities for Distribution of COVID-19 Vaccines*.
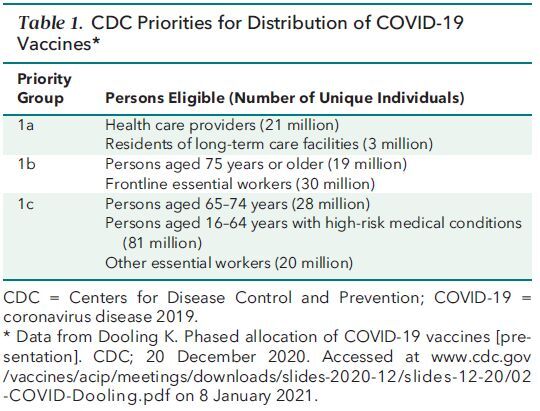
Table 2. Characteristics of the Most Advanced Vaccine Candidates for the United States*.
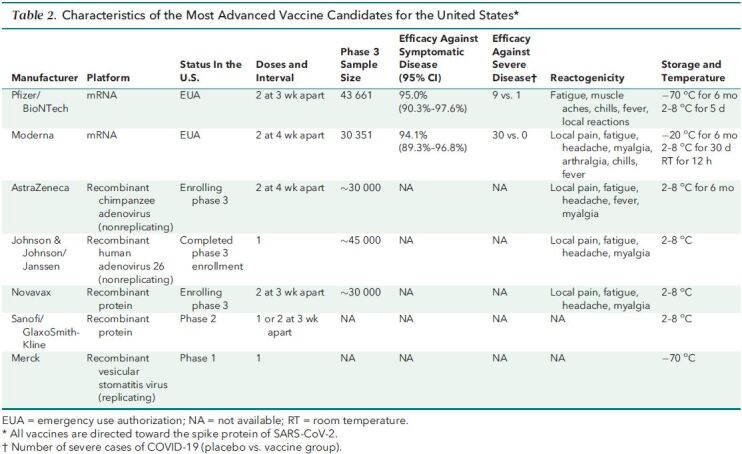
Figure. Cumulative incidence curves for the first COVID-19 occurrence after the first dose of mRNA vaccine.
A. Pfizer-BioNTech vaccine (BNT162b2). Figure reproduced from reference 2 (www.fda.gov/media/144245/download). B. Moderna vaccine (mRNA-1273). Individual severe COVID-19 data similar to those displayed in panel A are not publicly available. Figure reproduced from reference 4 (www.fda.gov/media/144434/download).
Following closely behind the mRNA vaccines are 2 vaccines undergoing testing that use adenoviral vectors: the AstraZeneca vaccine, using a chimpanzee-derived adenovirus (ChAdOx), and the Johnson & Johnson/Janssen vaccine, using a human adenovirus (Ad26). These are replication-incompetent viral vectors in which part of the viral DNA has been deleted and the DNA of the SARS-CoV-2 spike protein has been inserted. These vaccine vectors can enter host cells and express the spike protein but are unable to replicate. Given that there is substantial natural exposure to the approximately 70 types of circulating human adenoviruses, preexisting immunity to the vaccine platform from prior adenoviral infections could dampen the immune response to the spike protein antigens expressed by an adenoviral vaccine. Both adenoviral vaccines are designed to minimize this problem by selecting vectors with low seroprevalence in humans. Johnson & Johnson/Janssen has recently published interim results of their phase 1–2a study showing their approach is immunogenic (5) and recently completed enrollment in their approximately 45 000-volunteer phase 3 study. Initial results are expected in early 2021. AstraZeneca recently announced interim results of its international phase 3 trial and noted approximately 70% protection from clinical disease overall (6). Of note, those results were a composite of 2 different dosing regimens used in several countries. While efficacy was as high as 90% in a small cohort of participants in the United Kingdom who received a 50% lower first dose than the remainder of the study population and 62% in those who received the full dose for both the prime and the boost, it is unclear if this difference in efficacy is related to differences in dose, the interval between doses (4 to 12 weeks), or other confounding factors. Data from the AstraZeneca trial in the United States using a full dose for both the prime and the boost (at 28 days) are expected in early 2021.
There are many additional vaccine platforms in various stages of development around the world. Among the most advanced in testing in the United States are the spike protein vaccine produced by Novavax, which is in advanced phase 3 testing in the United Kingdom and early phase 3 testing in the United States, and the spike protein vaccine from Sanofi-GlaxoSmithKline, which is using Sanofi's established technology for producing influenza virus vaccines. Both the Novavax and Sanofi-GlaxoSmithKline proteins are produced in insect cells using recombinant baculovirus. In addition to these vaccines, Merck has begun phase 1 testing of an attenuated recombinant vesicular stomatitis virus expressing the spike protein. Each of these approaches has some unique attributes with regard to immunogenicity, cost, reactogenicity, ease of administration, and distribution logistics (such as cold chain requirements).
As the first wave of vaccines is now rolling out, health care providers need to be prepared to address questions regarding the safety of these vaccines. As large populations become vaccinated, it is possible for rare side effects to emerge. Although these vaccines have been administered to tens of thousands of people, very rare and serious side effects can be observed only after vaccination of millions of people. Also of note, in any large immunization campaign, many adverse events will be noted in individuals after vaccination given the fact that adverse events (for example, heart attack, stroke, and diabetes complications) occur every day in the absence of vaccination. It would be difficult to clearly attribute these temporally associated events to vaccination without better data pointing to a causal relationship. We will need to rely on careful epidemiologic evaluations of such events to sort out background morbidities within the population from the identification of new, rare side effects attributable to the vaccines. While most vaccine-related adverse events would be expected over the first few weeks to months after vaccination, the possibility remains that some could occur over the longer term. The logistics of collection and aggregation of such data will be highly complex given the number of vaccines that might be available by mid-2021, the number of shots required, and the diverse outlets planned for vaccination. Anyone suspecting an adverse event from vaccination is encouraged to report it to the Vaccine Adverse Event Reporting System (https://vaers.hhs.gov/reportevent.html).
Additional unknowns include the safety and efficacy of the vaccines in “special” populations, such as children, pregnant women, individuals with underlying illnesses, and those taking medications that might influence the immune response to a vaccine. The phase 3 trials were carefully designed to include participants who were diverse with regard to ethnicity, race, age and comorbidities; however, they typically excluded pregnant women, children, and those with immunodeficiency or a history of allergic reactions to vaccines. Although persons younger than 16 years were excluded from their phase 3 trials, both Pfizer-BioNTech and Moderna have plans to test their vaccines in pediatric populations. Additional studies are under way to examine safety and immunogenicity in larger groups of special populations. The recent examples of immediate hypersensitivity reactions in a number of recipients of the Pfizer-BioNTech and Moderna vaccines, some with a history of hypersensitivity reactions, highlight this point.
Another unknown is the duration of protection provided by these vaccines. Data were recently published on the serum antibody response to the Moderna vaccine out to 119 days after the first vaccination (90 days after the second immunization) (7). Although there was only an approximately 2-fold decline in antibody titers over this time, in prior trials of influenza virus vaccines using the mRNA and protein platforms, serum antibodies waned dramatically by 6 months to 1 year (8). At present, the durability of the immune response to the spike protein is unknown. It is possible that symptomatic or severe disease may be durably curtailed by memory T-cell and/or B-cell responses, however, this remains uncertain and the duration of protection needs to be carefully monitored.
Perhaps the unknown with the greatest potential immediate impact on the current pandemic is the degree to which these vaccines protect against infection and transmission. The high degree of protective efficacy reported thus far in these trials refers to symptomatic disease. Protection from actual infection may be considerably less, whereas protection from severe disease may be considerably higher. The ability of these vaccines to protect against infection is being analyzed by looking for evidence of asymptomatic infections in the vaccinated cohorts in the phase 3 trials through shedding of virus by asymptomatic individuals and development of antibodies to viral proteins not included in the vaccines. In preliminary data reported to the FDA by Moderna, 38 participants in the placebo group compared with 14 participants in the vaccine group were found to be shedding virus in the absence of symptoms before the second immunization, suggesting a degree of protection from infection and, by extension, decreased transmission. The distinction between immunity that protects a vaccinated person from developing symptomatic disease and immunity capable of also interrupting transmission of the virus from the vaccinated person to others is an important consideration for population immunity. This distinction is frequently lost in discussions about the collective societal responsibility to get vaccinated to reach an adequate level of population (herd) immunity to eliminate transmission. Failure to appreciate this distinction may lead to a false sense in vaccinees that they are protected from infection and thus cannot transmit to susceptible contacts. Hence, it is critical to continue to reinforce the public health measures of social distancing, handwashing, and masking until the current outbreak is under control.
Given that recent polling suggests that only 40% to 60% of people in the United States are currently planning to get vaccinated, it is conceivable that without some impact on transmission, the virus will continue to circulate, infect, and cause serious disease in certain segments of the unvaccinated population. Administration of parenterally administered vaccines alone typically does not result in potent mucosal immunity that might interrupt infection or transmission (9). In the case of poliovirus, induction of mucosal immunity through vaccination with the live attenuated oral polio vaccine, in contrast to the parenterally administered inactivated vaccine, was thought to have played a critical role in interruption of transmission and control of poliovirus epidemics (10). For these reasons, additional data regarding protection from infection should be generated as soon as possible. If these vaccines do not provide durable, high levels of protection from infection, and do not drive the prevalence of virus in the community to near zero, a thorough analysis of shedding and transmission will need to be done through additional study. Armed with such data, public health officials can make decisions regarding prioritization of populations to receive the vaccine, and researchers could potentially improve upon the first wave of vaccines.
Progress toward effective vaccines for SARS-CoV-2 has proceeded at an unprecedented pace. In the coming weeks and months, physicians must be prepared to explain the rapidly increasing body of data supporting the safety and efficacy of these vaccines, while at the same time acknowledging some degree of uncertainty. The FDA and CDC have provided thorough and thoughtful reviews leading to decisions that have been informed by public consultation and independent advisory panels. Answers to frequently asked questions are posted on their websites and frequently updated. The road ahead will almost certainly have plenty of bumps, and our current understanding of these vaccines is very likely to change over the coming months. It is incumbent on frontline professionals to be well informed about these vaccines to provide evidence-based recommendations to their patients on whether to be vaccinated. Health care providers should keep a watchful eye out for new information on safety, efficacy, and durability as it becomes available. It is highly likely that vaccination and its subsequent ability to prevent disease will provide critical and life-saving benefit in the coming months and may be one of our surest ways to emerge from this pandemic to a more normal society. Acknowledging that there is still much to learn while strongly encouraging vaccination is arguably one of the most critical challenges facing health care providers today. Having a clear understanding of the data supporting the use of these new vaccines is critical to addressing that challenge.
Footnotes
This article was published at Annals.org on 19 January 2021
References
- 1. Polack FP , Thomas SJ , Kitchin N , et al; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. [PMID: ] doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccines and Related Biological Products Advisory Committee Meeting, FDA Briefing Document: Pfizer-BioNTech COVID-19 Vaccine. U.S. Food and Drug Adminstration; 10 December 2020. Accessed at www.fda.gov/media/144245/download on 8 January 2021.
- 3. Baden LR , El Sahly HM , Essink B , et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020. [PMID: ] doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccines and Related Biological Products Advisory Committee Meeting, FDA Briefing Document: Moderna COVID-19 Vaccine. U.S. Food and Drug Administration; 17 December 2020. Accessed at www.fda.gov/media/144434/download on 8 January 2021.
- 5. Sadoff J , Le Gars M , Shukarev G , et al. Interim results of a phase 1-2a trial of Ad26.COV2.S covid-19 vaccine. N Engl J Med. 2021. [PMID: ] doi: 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M , Clemens SAC , Madhi SA , et al; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [PMID: ] doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Widge AT , Rouphael NG , Jackson LA , et al; mRNA-1273 Study Group. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination [Letter]. N Engl J Med. 2021;384:80-82. [PMID: ] doi: 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldman RA , Fuhr R , Smolenov I , et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326-3334. [PMID: ] doi: 10.1016/j.vaccine.2019.04.074 [DOI] [PubMed] [Google Scholar]
- 9. Russell MW , Moldoveanu Z , Ogra PL , et al. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11:611337. [PMID: ] doi: 10.3389/fimmu.2020.611337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hird TR , Grassly NC . Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. [PMID: ] doi: 10.1371/journal.ppat.1002599 [DOI] [PMC free article] [PubMed] [Google Scholar]