Abstract
Background
Modeling of the London hepatitis C virus (HCV) epidemic in men who have sex with men (MSM) and are living with human immunodeficiency virus (HIV) suggested that early access to direct-acting antiviral (DAA) treatment may reduce incidence. With high rates of linkage to care, microelimination of HCV within MSM living with HIV may be realistic ahead of 2030 World Health Organization targets. We examined trends in HCV incidence in the pre- and post-DAA eras for MSM living with HIV in London and Brighton, United Kingdom.
Methods
A retrospective cohort study was conducted at 5 HIV clinics in London and Brighton between 2013 and 2018. Each site reported all acute HCV episodes during the study period. Treatment timing data were collected. Incidence rates and reinfection proportion were calculated.
Results
A total of
378 acute HCV infections were identified, comprising 292 first infections and 86 reinfections. Incidence rates of acute HCV in MSM living with HIV peaked at 14.57/1000 person-years of follow-up (PYFU; 95% confidence interval [CI], 10.95–18.20) in 2015. Rates fell to 4.63/1000 PYFU (95% CI, 2.60 to 6.67) by 2018. Time from diagnosis to starting treatment declined from 29.8 (2013) to 3.7 months (2018).
Conclusions
We observed a 78% reduction in the incidence of first HCV episode and a 68% reduction in overall HCV incidence since the epidemic peak in 2015, which coincides with wider access to DAAs in England. Further interventions to reduce transmission, including earlier access to treatment and for reinfection, are likely needed for microelimination to be achieved in this population.
Keywords: hepatitis C, HIV, microelimination, directly acting antivirals
We examined acute hepatitis C virus (HCV) incidence in men who have sex with men and living with human immunodeficiency virus between 2013 and 2018 in London and Brighton, United Kingdom. Incidence rates peaked in 2015 at 14.57/1000 person-years of follow-up (95% confidence interval, 10.95 to 18.20). Following this there was a 68% reduction in HCV incidence by 2018, coinciding with wider access to direct-acting antivirals.
Hepatitis C virus (HCV) remains a major cause of morbidity and mortality throughout the world, responsible for approximately 600 000 deaths in 2015, the great majority from liver cancer and cirrhosis [1]. The estimated 2.3 million individuals living with human immunodeficiency virus (HIV) and HCV worldwide [2] are at increased risk of disease progression compared with those not living with HIV [3], and these differences are seen despite effective HIV treatment [4]. New interferon-free treatments for HCV with direct-acting antivirals (DAAs) have made highly effective and tolerable treatment more widely accessible to patients. These drugs have also demonstrated efficacy in the setting of acute HCV infection, with some clinical trials demonstrating high efficacy with shortened courses of treatment [5, 6].
This transformation in HCV treatment in recent years has contributed to ambitious World Health Organization (WHO) targets for elimination of HCV as a public health threat by 2030, including an 80% reduction in new HCV infections. Achieving these targets will require substantial effort and investment within a broad elimination strategy [7, 8]. The concept of microelimination has been proposed to break down wider population elimination targets into those focused on smaller subgroups for more targeted efforts [9]. Such an approach reflects the different patterns of risk for the disease across the globe and the different approaches required to address them. HIV cohorts are an important priority for microelimination [10, 11], and the British HIV Association has issued a microelimination statement aiming to treat all individuals living with both HIV and HVC in the United Kingdom by 2021 [12].
Previous phylogenetic work in the UK HIV population has suggested that more than 90% of acute HCV infections affect a small proportion of individuals with high-risk behaviors for HCV transmission [13]. Early modeling studies predicted that substantial scale-up of access to DAA therapy had the potential to reduce the prevalence of chronic infection in men who have sex with men (MSM) who are also living with HIV by more than 70% by 2025 [13, 14]. Real-world experience in other countries has suggested that such declines in HCV infection in MSM living with HIV can be seen where treatment access is good [15, 16]. However, a reduction in new HCV infections depends on a number of factors, including the extent to which those with active infection are engaged in care, the frequency and nature of screening at-risk populations, and access to DAA therapy. Declines in acute HCV infection have not been observed in all settings [15, 17, 18] and may be complicated by cross-linked populations including MSM who are not living with HIV and persons who inject drugs [19–21]
Here, our aim was to explore the impact of access to DAA therapy on the incidence of acute HCV infections among MSM living with HIV and engaged with HIV services in London and Brighton, United Kingdom.
METHODS: Treatment Access for Hepatitis C
DAA treatment became available via the National Health Service England (NHSE) treatment program in late 2015 for chronic HCV infection, with decompensated cirrhotic patients given priority. Access to treatment for milder disease stages began on 1 April 2016, with monthly regional treatment allocations determined nationally according to estimated prevalence and adjusted at intervals with individual centers. Centers were required to prioritize patients for treatment based on clinical need. The method for prioritization differed between centers. In general, those with more advanced fibrosis (stage F3/4) were prioritized for treatment. DAA treatment for acute (<6 months persistent viremia) infection and a second course of DAAs for reinfection were not permitted in this program during the study period, outside of clinical trials. All centers participated in DAA treatment clinical trials for acute and chronic noncirrhotic HCV/HIV patients throughout the study period, subject to availability. In addition, since early 2015, some MSM living with HIV had been accessing DAA generic medication via the Internet. All involved centers provided safety and virological monitoring support for patients who selected this approach. Additionally, all centers promoted risk-reduction behavior interventions. At some sites, this included clinical psychology assessments, motivational interviews, substance-misuse support, peer-support workshops, and contact tracing.
Setting
HIV clinics with large MSM cohorts were invited to participate based on their ability to provide a core dataset of routinely collected individual data for MSM living with HIV for the period July 2013 through June 2018. Data were provided by 4 HIV treatment centers across London (Royal Free NHS Trust, Imperial College Healthcare NHS Trust, Mortimer Market Centre, and Barts Health NHS Trust) and 1 in Brighton (Brighton & Sussex University Hospitals NHS Trust). National HIV testing guidelines were followed in all centers, which included at least annual HCV testing for all patients plus testing every 3 to 6 months for high-risk patients (using HCV antibody, HCV antigen, or HCV RNA testing according to local policy, HCV history, and risk assessment). After completing DAA therapy, patients had their HCV RNA checked every 1, 3, and 6 months following the end of treatment. In addition, patients had liver function tests (including alanine aminotransferase [ALT]) performed at 6-month intervals.
Definition of Acute HCV
A new episode of acute HCV infection was defined according to previously established criteria [17] as a positive HCV RNA test in the presence of a negative anti-HCV test within the past 12 months or a positive HCV RNA test with an acute ALT rise and no other identifiable cause. HCV reinfection was defined [18] as a positive HCV RNA test in patients who had previously achieved spontaneous clearance (anti-HCV positive individuals with 2 consecutive negative HCV RNA results 24 weeks apart and did not receive treatment), sustained virological response following treatment (negative HCV RNA result 24 weeks for interferon [IFN]-based or 12 weeks for DAA after stopping treatment or later), or with evidence of genotype switch and potential exposure.
Data Collection
Patient data that were collected included HIV parameters at the time of each acute HCV episode (HIV RNA, CD4+ cell count, whether currently receiving antiretroviral therapy [ART]), HCV parameters at the time of an acute HCV episode (HCV RNA, HCV genotype, peak ALT), details of the HCV treatment pathway (spontaneous clearance, IFN/ribavirin [RBV] with or without telaprevir/boceprevir, DAAs, clinical trial, generic medications, or not treated), time (months) from HCV diagnosis to starting treatment, and treatment outcome if applicable.
Each site reported all acute HCV episodes (first episode and reinfections) during the study period. For each 6-month period, additional data were collected, including the number of MSM living with HIV under active follow-up (attended an HIV appointment within the past 12 months) and the number and type of clinic-prescribed HCV/HIV treatments (including pegylated [PEG] IFN/RBV or DAAs).
Analyses
The incidence rate (IR) of all acute HCV infections and first acute HCV infections was calculated per 1000 MSM living with HIV person-years of follow-up (PYFU). An IR for HCV reinfection was not calculated due to practicalities of calculating an accurate and time-updated denominator of all MSM living with HIV with past HCV (HCV antibody positive and RNA negative) in a combined retrospective cohort of more than 9000 patients.
RESULTS
Incidence
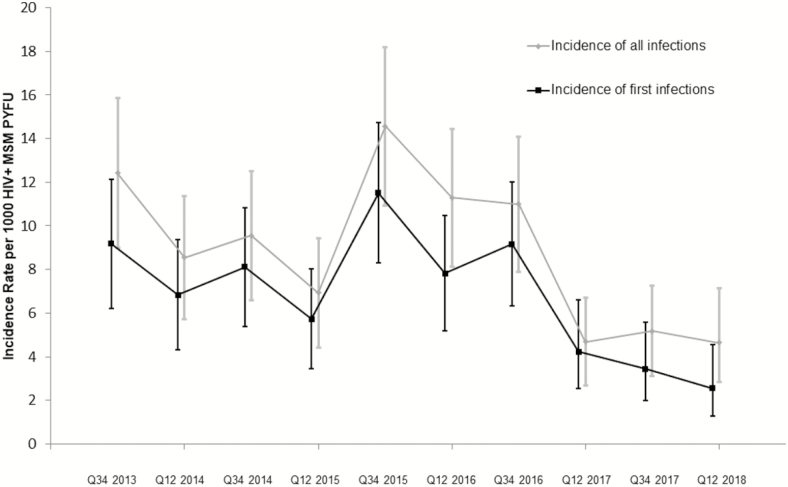
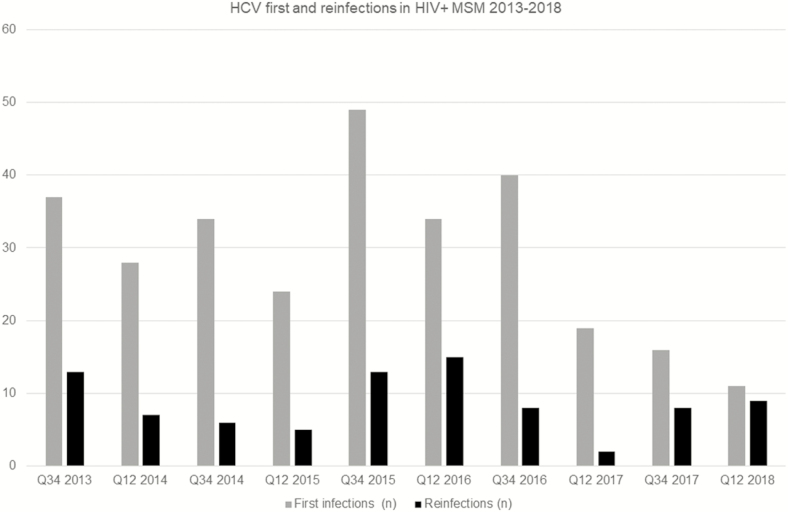
Between July 2013 and June 2018, the 5 centers contributed 42 899 MSM living with HIV PYFU data from 9278 MSM living with HIV for analysis. All centers reported cohort size, deaths, care transfers, and patients lost to follow-up annually. Between 2013 and 2018, the overall total MSM living with HIV cohort size increased by 7.2% (8053 to 8630). Transfers in and out of cohorts were stable and low at <4% per year. During the study period, 378 acute HCV infections were identified, comprising 292 first infections and 86 reinfections (Figure 1). Incidence rates of acute HCV in MSM living with HIV peaked at 14.57/1000 PYFU (95% confidence interval [CI], 10.95 to 18.20] in the second half of 2015. Rates fell to 4.63/1000 MSM living with HIV PYFU (95% CI, 2.60 to 6.67) by 2018 (Figure 2). The incidence of the first HCV episode also peaked in 2015 at 11.52/1000 PYFU (95% CI, 8.29 to 14.74). Rates fell to 2.55/1000 PYFU (95% CI, 1.04 to 4.06) by 2018.
Figure 1.
Incidence of acute hepatitis C virus infections, 2013 and 2018. Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; PYFU, person-years of follow-up. Abbreviation: Q, quarter.
Figure 2.
Hepatitis C virus first infections and reinfections in men who have sex with men living with human immunodeficiency virus, 2013–2018. Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; MSM, men who have sex with men; Q, quarter.
Patient Characteristics
All first and subsequent acute HCV diagnoses were treated as distinct episodes. At the time of an acute HCV episode, the median age was 43 years (interquartile range [IQR], 35–48] and the median CD4 + cell count was 650 (IQR, 480 830) cells/µL. A total of 338 patients (89%) were receiving ART and 310 (82%) had HIV RNA <50 copies/mL at the time of the acute HCV episode (increasing during the study period from 68% in 2013 to 95% in 2018). Individual clinical parameters are summarized in Table 1. Characteristics including patient age, HIV treatment status, and virological suppression rates were highly consistent with national and regional data surveillance reports [22].
Table 1.
Human Immunodeficiency Virus and Hepatitis C Virus (HCV) Clinical Parameters at Time of Acute HCV Episode
| Clinical Parameter | All Acute HCV Episodes | First Acute HCV Infection | Acute HCV Reinfection |
|---|---|---|---|
| Total number | 378 | 292 | 86 |
| Age, median (IQR), y | 43 (35–48) | 42 (25–48) | 44 (37–50) |
| Plasma CD4 + cell count, median (IQR) | 650 (480–830) | 644 (480–813) | 667 (503–858) |
| Receiving antiretroviral therapy at time of HCV episode, n (%) | 338 (89) | 257 (88) | 81 (94) |
| Human immunodeficiency virus RNA <50 copies/mL at time of HCV episode, n (%) | 310 (82) | 235 (81) | 75 (87) |
| HCV genotype | 281 (74.3) | 213 (72.9) | 68 (79.0) |
| 1 | 2 (0.5) | 2 (0.7) | 0 (0) |
| 2 | 31 (8.2) | 24 (8.2) | 7 (8.1) |
| 3 | 48 (12.7) | 39 (13.4) | 9 (10.5) |
| 4 | 16 (4.2) | 14 (4.8) | 2 (2.3) |
| Not known | |||
| HCV RNA, median (IQR) | 1 202 115 (118 115–7 824 998) | 1 800 000 (176 244–8 993 908) | 380 091 (18 428–1 775 217) |
| Peak ALT during HCV episode, median (95% confidence interval) | 350 (156 to 840) | 384 (163 to 900) | 305 (147 to 498) |
| Number of previous HCV episodes (range) | … | … | 1 (1–3) |
| Number of months elapsed since SVR/spontaneous clearance confirmed (IQR) | … | … | 22 (9–49) |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; IQR, interquartile range; SVR, sustained virological response.
HCV Treatment
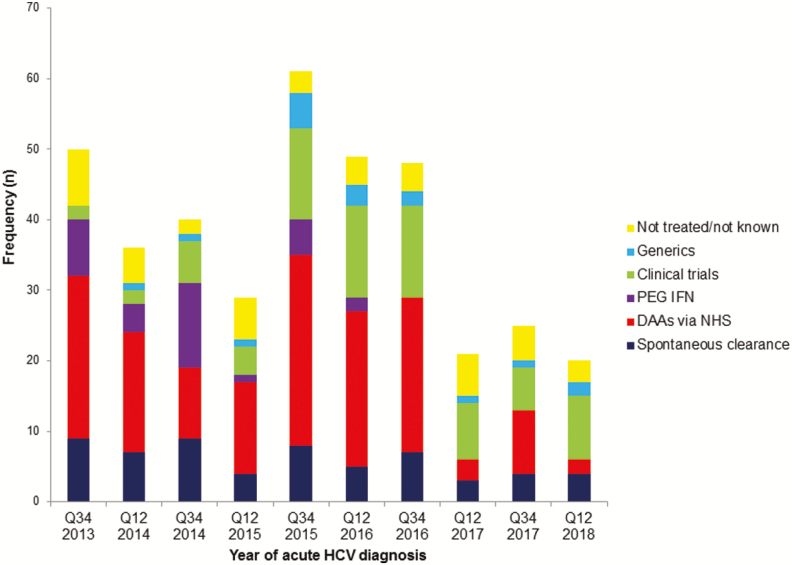
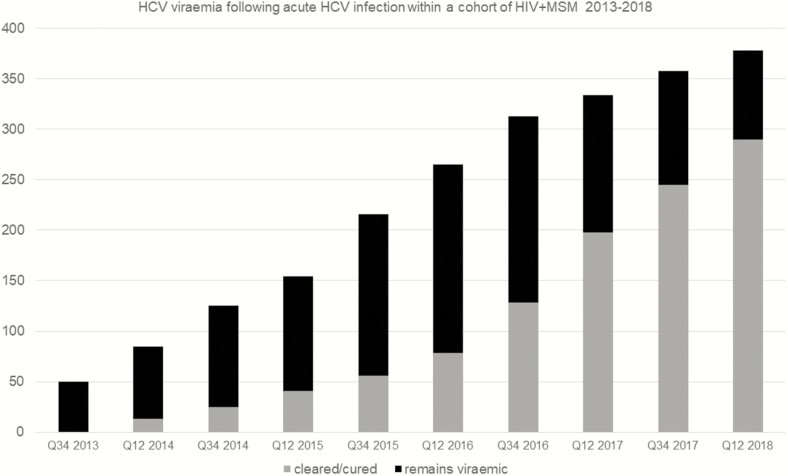
Of 378 acute HCV episodes between 2013 and 2018, 59 (15.6%) spontaneously cleared HCV. Of the remainder, 32 (8.5%) were treated with PEG IFN/RBV-based regimens, 147 (38.9%) were treated with DAAs via the NHSE program after chronic HCV infection was established (HCV RNA positive for at least 6 months), 17 (4.5%) self-purchased generic medications, 76 (20.1%) entered HCV clinical trials, 27 (7.1%) remained untreated, and 20 (5.3%) were HCV treatment status unknown and lost to follow-up at the end of the study period (Figure 3). The time from acute HCV diagnosis to the start of any HCV treatment declined from an average of 29.8 months (2013) to 3.7 months (2018). Shorter times to start treatment were observed most frequently in those who accessed HCV clinical trials. The estimated proportion of HCV viremia within this cohort declined during the study period, as patients completed treatment earlier (Figure 4).
Figure 3.
Treatment pathway undertaken according to year of acute HCV diagnosis, 2013–2018. Abbreviations: DAAs, direct-acting antivirals; HCV, hepatitis C virus; NHS, National Health Service; PEG IFN, pegylated interferon. Abbreviation: Q, quarter.
Figure 4.
Hepatitis C virus (HCV) viremia following acute HCV infection within a cohort of men who have sex with men living with human immunodeficiency virus, 2013–2018. Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; Q, quarter.
DISCUSSION
In a longitudinal cohort drawn from 5 large UK centers that provide regular HIV care to more than 9000 MSM living with HIV, we observed significant declines in the incidence of acute HCV infection following access to DAA therapy. As with the Swiss cohort [23], the incidence of acute HCV infection in MSM seemed to peak in 2015. In part, this likely reflects a steadily rising incidence over the previous decade, a trend that modeling studies predicted would continue in the absence of any new intervention [13]. However, it is also possible that a reduction in rates of PEG IFN-based treatments (see Figure 3) in anticipation of DAAs becoming available, referred to as “warehousing” [24, 25], potentially prolonged viremia and increased transmission. While not captured in this study, it is also possible that improved risk-reduction strategies in HIV clinics and workshops to promote awareness in chemsex (use of drugs in a sexual context) users also contributed to the decline in HCV incidence. However, this is not reflected in a decline in other sexually transmitted infections among MSM in these regions during the same period [26].
Declines in the incidence of acute HCV from 14.57/1000 PYFU in 2015 to 4.63/1000 (95% CI, 2.6 to 6.7) by 2018 coincided with the availability of DAA therapies from 2016 through the NHS, a single public sector health provider that offers universal coverage to those in need of treatment. It is likely these simultaneous declines in incidence are due to the prompt treatment of patients living with both HIV and HCV who were highly engaged with services and prepared to start DAAs as soon as able. It is likely that subsequent wider availability of DAAs (for all disease stages) the following year, plus more numerous DAA clinical trials for acute and early noncirrhotic HCV infection, contributed to the further declines in incidence that followed in 2016–2017. While it is possible that individuals who purchased generic treatment from buyer’s clubs (similar to that seen with community-driven access to pre-exposure prophylaxis [PrEP]), a widely reported phenomenon [27], also played a role. The absolute numbers of those who accessed treatment via this route were relatively small in these centers (n = 17 starting between 2015 and 2018). It may also be possible that individuals purchased generic medications without informing their clinicians and thus were miscategorized as spontaneous clearance. However, given clinic policies for supporting and monitoring individuals who select generics, it is unlikely many treatments were concealed.
There are clear limitations to the conclusions that can be drawn from this study. Only 1 of the centers studied is outside London (Brighton), and trends at other national clinics may be different. Of particular relevance, the access to enrollment in acute HCV trials enabled many individuals to access early treatment and is unlikely to represent all settings. Data were collected retrospectively and were not part of a formal study process, thus there is a possibility that incident infection may not have been ascertained equally during all periods across centers. However, all centers have dedicated coinfection services, and both testing practices and ascertainment are unlikely to have changed significantly over time. The availability of DAAs has raised the profile of HCV among MSM living with HIV as well as providers and may have been expected to increase the diagnosis of acute HCV, as opposed to the declines seen here. In order to provide a standardized denominator between clinics, the analysis was limited to MSM living with HIV, so we cannot comment on the incidence of HCV in other closely related high-risk groups, for example, those who actively inject drugs and/or individuals not living with HIV who are on PrEP and in whom a high incidence of HCV has been reported [28].
The data demonstrate that HCV reinfection in UK MSM living with HIV remains challenging; however, the absolute number of reinfections remained fairly consistent during the study period, despite an increasing population of previously cleared/cured individuals (Figure 4). Reinfection is a particular health concern within services currently supported by NHSE. Early treatment of HCV infection, which has been shown to be both effective and cost-effective [29–31], is not permitted until beyond 6 months of persisting viremia (at the time of writing), nor is a second course of DAA treatment for reinfection. Furthermore, the date of infection can predate the diagnosis by months, even with regular testing protocols, resulting in lengthy periods of asymptomatic viremia while awaiting treatment approval. Requiring a minimum of 6 months HCV viremia for chronic infection to be determined contrasts with European guidelines, which recommend chronic infection be determined based on week 4 HCV RNA viral log decline plus/minus the week 12 RNA level if required [32]. Earlier determination of chronic infection and treatment initiation would reduce the duration of viremia by several weeks as well as the potential for onward transmission.
Our data provide evidence that significant declines in acute HCV incidence can be achieved. However, we have concerns that, following the warehousing effect and subsequent sharp fall, we are now observing an incidence plateau. The results still fall short of WHO targets for reductions in new diagnoses that, based on our data, would be equivalent to 1.5 per 1000 MSM living with HIV PYFU. We propose that this should be the incidence target that all national HIV services should aim to achieve. Whether this target can lead to microelimination needs to be assessed in the context of local epidemiology. For example, data from the Dutch cohort [16] suggest the target should be lower still, at 1.1 per 1000 MSM living with HIV PYFU. Ideally, in the future, surveillance data will be collected from MSM living with HIV populations, particularly those receiving PrEP to support the development of microelimination targets for this population. Our data suggest that access to earlier public sector treatment than is currently available, including for reinfection, will remain important to ensure progress to microelimination targets in MSM living with HIV.
Notes
Author contributions. L. J. G., G. S. C., S. B., C. Smith, A. R.: study design, literature review, data collection, data interpretation, and manuscript draft and approval. L. J. G., C. Smith: data analysis. C. Stingone, I. G., L. J., L. J. W., T. M., F. F., C. Sood, C. F., C. P., R. B., H. S., Y. G., A. B., C. O., S. U.: data collection and manuscript draft and approval.
Acknowledgments. The authors thank all patients and clinic staff who contributed to this study.
Financial support. The work was supported, in part, by the Biomedical Research Centre of the Imperial College National Health Service Trust and an National Institute for Health Research Professorship awarded to G. C.
Potential conflicts of interest. A. B. reports grants from Gilead and personal fees from AbbVie and Merck Sharp & Dohme (MSD). G. S. C. has provided consultancy for Gilead Inc and lectured for MSD. L. J. G. reports advisory/speaker fees or conference support from Gilead and Janssen. L. J. W. reports advisory/speaker fees or conference support from Gilead, ViiV, Janssen, MSD, Cipla, and Mylan. Y. G. reports advisory/speaker fees or conference support from Gilead, ViiV, Janssen, and MSD. C. Smith reports personal fees for preparation of educational materials from Gilead Sciences and ViiV. C. O. reports research funding to Institution for investigator-led clinical trials from Gilead, MSD, ViiV, GlaxoSmithKline (GSK), and Janssen and reports advisory/speaker fees and travel scholarships from Gilead, MSD, ViiV, GSK, and Janssen. I. G. reports conference support from Gilead. S. B. reports speaker fees and advisory board fees from AbbVie and Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 3. Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–6. [DOI] [PubMed] [Google Scholar]
- 4. Lo Re V 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 2014; 160: 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017; 2:347–53. [DOI] [PubMed] [Google Scholar]
- 6. Boerekamps A, De Weggheleire A, van den Berk GE, et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, phase 3b trial. Lancet Gastroenterol Hepatol 2019; 4:269–77. [DOI] [PubMed] [Google Scholar]
- 7. Cooke GS, Andrieux-Meyer I, Applegate TL, et al. ; Lancet Gastroenterology & Hepatology Commissioners Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019; 4:135–84. [DOI] [PubMed] [Google Scholar]
- 8. Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 2019; 393:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazarus JV, Wiktor S, Colombo M, Thursz M; EASL International Liver Foundation Micro-elimination— A path to global elimination of hepatitis C. J Hepatol 2017; 67:665–6. [DOI] [PubMed] [Google Scholar]
- 10. Lazarus JV, Safreed-Harmon K, Thursz MR, et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis 2018; 38:181–92. [DOI] [PubMed] [Google Scholar]
- 11. Microelimination could be a big deal for HCV and HIV services. The Lancet HIV 2018; 5:e605. [DOI] [PubMed] [Google Scholar]
- 12. BHIVA. BHIVA calls for accelerated efforts to prevent and cure hepatitis C infection 2018. https://http://www.bhiva.org/BHIVA-calls-for-accelerated-efforts-to-prevent-and-cure-hepatitis-C-infection. Accessed 9 August 2019.
- 13. Martin NK, Thornton A, Hickman M, et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis 2016; 62:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57(Suppl 2):S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boerekamps A, Newsum AM, Smit C, et al. ; NVHB-SHM Hepatitis Working Group and the Netherlands ATHENA HIV Observational Cohort High treatment uptake in human immunodeficiency virus/hepatitis C virus-coinfected patients after unrestricted access to direct-acting antivirals in the Netherlands. Clin Infect Dis 2018; 66:1352–9. [DOI] [PubMed] [Google Scholar]
- 16. Boerekamps A, Van den Berk GE, Fanny LN, et al. Declining HCV incidence in Dutch HIV positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis 2018; 66:1360-5. [DOI] [PubMed] [Google Scholar]
- 17. Pradat P, Huleux T, Raffi F, et al. ; Dat’AIDS Study Group Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS 2018; 32:1077–82. [DOI] [PubMed] [Google Scholar]
- 18. Sulkowski MS. The proof is in the patient: hepatitis C virus microelimination in the Swiss Human Immunodeficiency Virus Cohort Study. Clin Infect Dis 2019; 68:577–9. [DOI] [PubMed] [Google Scholar]
- 19. Boesecke C, Grint D, Soriano V, et al. ; EuroSIDA in EuroCoord Hepatitis C seroconversions in HIV infection across Europe: which regions and patient groups are affected? Liver Int 2015; 35:2384–91. [DOI] [PubMed] [Google Scholar]
- 20. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. ; Amsterdam PrEP Project Team in the HIV Transmission Elimination AMsterdam Initiative, MOSAIC Study Group MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017; 31:1603–10. [DOI] [PubMed] [Google Scholar]
- 21. Charre C, Cotte L, Kramer R, et al. Hepatitis C virus spread from HIV-positive to HIV-negative men who have sex with men. PLoS One 2018; 13:e0190340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Public Health England. Prevalence of HIV infection in the UK in 2018. Health Protection Report. 2019; 13 Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843766/hpr3919_hiv18.pdf. Accessed 30 January 2020. [Google Scholar]
- 23. Salazar-Vizcaya L, Wandeler G, Fehr J, et al. ; Swiss HIV Cohort Study Impact of direct-acting antivirals on the burden of HCV infection among persons who inject drugs and men who have sex with men in the Swiss HIV Cohort Study. Open Forum Infect Dis 2018; 5:ofy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owens GM. Revolutionizing treatment outcomes in hepatitis C: managed care implications and considerations— the new and evolving standards of care. Am J Manag Care 2015; 21:S97–105. [PubMed] [Google Scholar]
- 25. Palak A, Livoti C, Audibert C. Considerations on bringing warehoused HCV patients into active care following interferon-free, direct-acting antiviral drug approval. Postgrad Med 2017; 129:471–5. [DOI] [PubMed] [Google Scholar]
- 26. PHE. STI diagnoses and rates in England by gender, 2008 to 2017 2018. https://http://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables. Accessed 9 August 2019.
- 27. Hill A, Khwairakpam G, Wang J, et al. High sustained virological response rates using imported generic direct acting antiviral treatment for hepatitis C. J Virus Erad 2017; 3:200–3. [PMC free article] [PubMed] [Google Scholar]
- 28. Cotte L, Cua E, Reynes J, et al. Hepatitis C virus incidence in HIV-infected and in preexposure prophylaxis (PrEP)-using men having sex with men. Liver Int 2018; 38:1736–40. [DOI] [PubMed] [Google Scholar]
- 29. Popping S, Hullegie SJ, Boerekamps A, et al. Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men. PLoS One 2019; 14:e0210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European ATNAHCICP. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. Aids 2011; 25:399–409. [DOI] [PubMed] [Google Scholar]
- 31. EASL. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 32. EACS. Guidelines Version 9 2017http://www.eacsociety.org/files/guidelines_9.0-english.pdf. Accessed 9 August 2019.