Fig. 3. Conformations of CP.
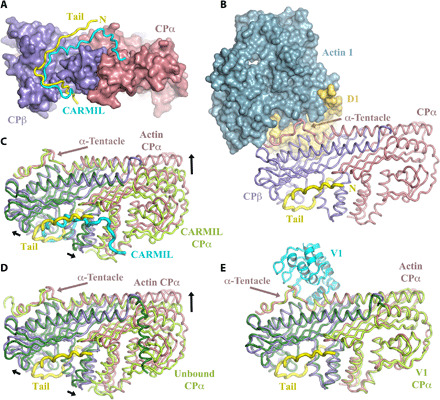
(A) Comparison of the twinfilin tail (yellow) binding site with CARMIL (cyan) on CP. Both CP-binding peptides run in the same direction, and N terminus of the twinfilin tail is labeled N. (B) The actin-binding site on the CP α-tentacle is distant from the twinfilin tail-binding site. (C and D) Structural superimposition of the β-subunits of CP reveals that the conformation of CP in the twinfilin/CP/actin complex is different to the CARMIL-bound and unbound conformations of CP. (E) Superimposition reveals that the conformation of CP in the twinfilin/CP/actin complex is similar to the V-1–bound conformation of CP. α- and β-subunits of CP are colored light green and dark green, respectively, for the CARMIL, unbound, and V-1 complexes. Black arrows indicate conformational changes in CP in adopting the twinfilin/CP/actin complex structure.
