Figure 3: Cytoprotective-selective 3K3A-APC.
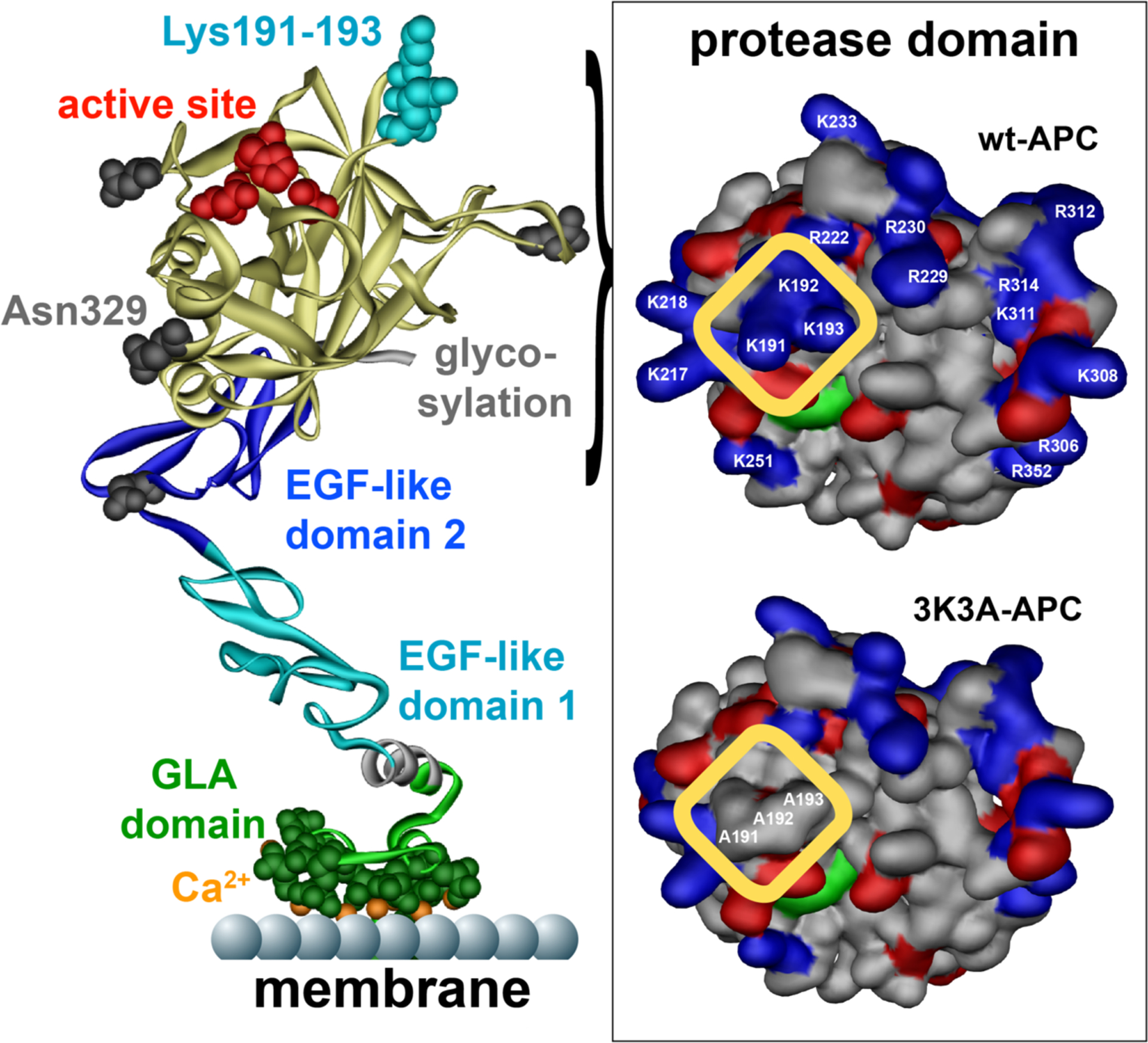
APC, denoted on the left by a ribbon structure, is comprised of an N-terminal GLA domain (green) that binds to negatively charged phospholipids and EPCR, two EGF-like domains (blue), and the protease domain (yellow) containing the active site (red). Four glycosylation attachment sites are indicated in gray. Interaction of APC with its multiple substrates is often mediated by charged residues in surface exposed loops on APC. These so called binding exosites on APC vary for different substrates. Three positively charged lysine residues (KKK191–193) in the 37-loop of APC form a positively charged patch (see highlighted yellow box top right) important for APC’s recognition of factors Va and VIIIa. Mutation of these 3 lysine residues to alanine (3K3A-APC) eliminates this binding exosite for factors Va and VIIIa on the surface of APC (see highlighted yellow box bottom right) and results in a reduction of APC’s anticoagulant activity by 90%. In contrast, the cytoprotective substrates, PAR1, or its other known cell signaling receptors do not require these 3 lysine residues for interaction with APC and consequently 3K3A-APC retains normal cytoprotective activities. While surfaces on APC interacting with PAR1 remain to be fully defined, a potential binding exosite for PAR1 is located in the protease domain of APC (yellow) around the glycosylation attachment site at Asn329 (bottom left). Elimination of the glycosylation at Asn329 increases APC’s cytoprotective activities, whereas mutation of two nearby negatively charged glutamic acid residues at 330 and 333 to alanine eliminates APC’s cytoprotective activities.126,127 This suggests that exosites for APC’s anticoagulant and cytoprotective substrates are located on different and opposite sides of the protease domain thereby providing an explanation why 3K3A-APC is cytoprotective-selective. This figure is modified from Griffin et al, Blood, 2018.2
