Abstract
Organisms are often exposed to fluctuating environments and changes in intracellular homeostasis, which can have detrimental effects on their proteome and physiology. Thus, organisms have evolved targeted and specific stress responses dedicated to repair damage and maintain homeostasis. These mechanisms include the unfolded protein response of the endoplasmic reticulum (UPRER), the unfolded protein response of the mitochondria (UPRMT), the heat shock response (HSR), and the oxidative stress response (OxSR). The protocols presented here describe methods to detect and characterize the activation of these pathways and their physiological consequences in the nematode, C. elegans. First, the use of pathway-specific fluorescent transcriptional reporters are described for rapid cellular characterization, drug screening, or large-scale genetic screening (e.g. RNAi or mutant libraries). In addition, complementary, robust physiological assays are described, which can be used to directly assess sensitivity of animals to specific stressors, serving as functional validation of the transcriptional reporters. Together, these methods allow for rapid characterization of the cellular and physiological effects of internal and external proteotoxic perturbations.
Keywords: Stress, C. elegans, unfolded protein response, endoplasmic reticulum, mitochondria, heat shock response, transcriptional reporter, oxidative stress, protein homeostasis
SHORT ABSTRACT:
Characterization of cellular proteotoxic stress responses in the nematode C. elegans by measuring the activation of fluorescent transcriptional reporters and assaying sensitivity to physiological stress.
INTRODUCTION:
The ability of an organism to respond to changes in the intra- and extracellular environment is crucial for its survival and adaptation. This is accomplished on a cellular level through numerous protective pathways that ensure the integrity of the cell. While numerous cellular components are subject to stress-associated damage, one major involvement of cellular stress responses is to repair and protect the homeostasis of the cellular proteome. However, the compartmentalization of proteins into special structures, called organelles, poses a challenge for the cell, as it cannot rely on one centralized form of protein quality control to ensure all the proteins within the cell are properly folded and functional. Therefore, to deal with perturbations to their proteins, organelles have evolved dedicated quality control mechanisms, which can sense misfolded proteins and activate a stress response in an attempt to alleviate the stress within that compartment. For example, the cytosol relies on the heat shock response (HSR), while the endoplasmic reticulum (ER) and mitochondria rely on their compartment-specific unfolded protein responses (UPR). The OxSR serves to alleviate the toxic effects of reactive oxygen species (ROS). Each stress response is triggered in the presence of cellular challenges and environmental insults, and induces a tailored transcriptional response. The hallmark of these responses include synthesizing molecules that re-fold misfolded proteins (such as chaperones) targeted to the proper organelle, or alternatively, remove damaged proteins by protein degradation. Failure to activate these stress responses results in accumulation of damaged proteins, cellular dysfunction propagated to systemic failure of tissues, and eventually death of the organism. The function and regulation of the different stress responses are reviewed elsewhere1.
Many insights regarding the regulation and activity of cellular stress responses have been attributed to the nematode, Caenorhabditis elegans, a multicellular model organism in genetic research. Nematodes not only allow studying the activation of stress responses on the cellular level, but also on the organismal level; nematodes have been used to study the effects of genetic perturbations or exposure to drugs and pollutants on their growth and survival. Their quick generation time, isogeny, transparency, genetic tractability, and ease of use during experimentation make them ideal for such studies. Additionally, the relatively quick physiological response to stress (between hours and a few days) and the evolutionary conservation of cellular pathways make nematodes a prominent tool in studying stress resistance.
There are two commonly used E. coli strains used as a food source to grow C. elegans: standard OP50, a B strain in which most experimentation has been historically performed2 and HT115, a K-12 strain that is used for almost all RNAi experiments3, 4. It is important to note that there are significant differences between OP50 and HT115 bacterial diets. Growth on these different bacterial sources has been shown to cause major differences in metabolic profile, mitochondrial DNA copy number, and several major phenotypes, including lifespan5. Some of these differences are attributed to Vitamin B12 deficiency associated with growth on OP50 bacteria, which can result in defects in mitochondrial homeostasis and increased sensitivity to pathogens and stresses. All of these phenotypes have been shown to be alleviated by growth on HT115 bacteria, which have higher levels of Vitamin B126. Therefore, it is recommended that all experiments on physiological stress responses be performed on HT115 bacteria, regardless of the necessity of RNAi conditions. However, due to the ease of maintaining animals on OP50, all standard growth (i.e. maintenance and amplification of animals) can be performed on OP50, as significant differences in the experimental paradigms described here were not detected in worms maintained on OP50 as long as they were moved to HT115 post synchronization (i.e. from hatch post-bleaching with or without L1 arresting) until experimentation.
Here, the characterization of the activity of cellular stress responses using two functional methods is described. It should be noted that the protocols presented are primarily focused on cellular stress responses and their impact on protein homeostasis. First, fluorescent transcriptional reporters are utilized, which are regulated by endogenous gene promoters that are specifically activated in response to different cellular stresses. These fluorescent transcriptional reporters are based on the transcriptional induction of specific genes that are natively part of the stress response: For example, HSP-4, a heat shock protein orthologous to the human chaperone HSPA5/BiP, is activated upon ER-stress and localizes to the ER to alleviate the stress. In conditions of ER stress (for example, exposure to tunicamycin), a green fluorescent protein (GFP), placed under the regulation of the hsp-4 promoter, is synthesized in high levels as can be assessed by fluorescent microscopy or quantitatively measured using large-particle flow cytometry of nematodes7. Similarly, the promoter of a mitochondrial chaperone, hsp-6 (orthologous to mammalian HSPA9), is utilized to monitor the activation of the UPRMT8, and the promoter of the cytosolic chaperone hsp-16.2 (orthologous to the human crystallin alpha genes) is used for assessing the activity of the HSR9. These reporters allow a rapid characterization of the pathways activated in response to various perturbations.
Often, the reporters presented here are imaged using microscopy, which provides a qualitative output of the activation of stress responses. However, while imaging techniques provide both information on intensity and tissue location of the reporters described above, its quantification is not always accurate or robust. While it is possible to quantify fluorescent activation using imaging analysis tools, these methods are relatively low throughput and sample size is small, due to the relatively low number of animals imaged. The ease and ability to obtain large quantities of animals quickly make C. elegans an ideal model system to assay the activation of fluorescent stress reporters through the use of a large particle flow cytometer. A large-particle flow cytometer is capable of recording, analyzing, and sorting based on size and fluorescence from many live animals. Using this method, it is possible to get the fluorescent intensity, size, and also spatial (2D) information for thousands of worms. The system is controlled using FlowPilot, which allows for real-time data acquisition and analysis of the measured parameters. Here, methods for both microscopic imaging and quantitative analysis using a large-particle flow cytometer are offered as methods to measure the activation of stress responses.
Beyond reporter analysis, the sensitivity or resistance of animals to stress can be measured using physiological stress assays. This is achieved by exposing animals to stressful environments that activate specific cellular stress pathways. Here, several methods are provided to measure sensitivity of whole animals to specific types of stressors.
ER stress is applied to C. elegans by using the chemical agent, tunicamycin, which blocks N-linked glycosylation, causing accumulation of misfolded proteins in the ER10. In C. elegans, growth upon exposure to tunicamycin results in major perturbations in ER function, and a significantly decreased lifespan11. By measuring the survival of animals on tunicamycin-containing plates, ER stress sensitivity of animals can be quantified. For example, animals with ectopic UPRER induction and thus increased resistance to protein misfolding stress in the ER have an increased survival upon tunicamycin exposure compared to wild-type animals12.
Oxidative and mitochondrial stress is applied to C. elegans by exposing animals to the chemical agent, paraquat. Paraquat is a commonly used herbicide, which causes superoxide formation specifically in the mitochondria13. Due to the specific localization of mitochondria-derived reactive oxygen species (ROS), paraquat assays are often used as a “mitochondrial” stress assay. However, superoxide is rapidly converted into hydrogen peroxide by mitochondrial superoxide dismutases (SODs)14. Hydrogen peroxide can subsequently diffuse out of the mitochondria and cause oxidative stress in other compartments of the cell. Therefore, we describe paraquat survival assays as measuring sensitivity to both mitochondrial and oxidative stress (other oxidative stress assays can be found15).
Thermotolerance assays are performed in C. elegans by placing animals in elevated temperatures. Ambient temperatures for nematodes are ~15-20 °C and thermal stress is induced at temperatures above 25 °C16, 17. Thermotolerance assays are generally performed at temperatures ranging from 30-37 °C, as animals exhibit major cellular defects at this temperature, and survival assays are completed within 24 hours16, 18. Here, two alternative methods are provided for performing thermotolerance assays: growth at 34 °C and growth at 37 °C. Together, the protocols presented here can be utilized to perform large-scale screens when combined with standard gene knock-down using RNA interference or chemical drug libraries.
PROTOCOL:
1. Growth of C. elegans and preparation for imaging.
1.1. Standard growth conditions of temperatures & OP50 vs HT115.
Standard growth and expansion
1. Grow a culture of OP50 in LB (see Table 1) or equivalent media of choice for 24-48 hours in ambient temperature (~22-25 °C). It is recommended to grow bacteria in room temperature as OP50 is a uracil auxotroph and there is a higher incidence of revertants (e.g. suppressor mutants) when grown at 37 °C. Long-term storage of OP50 cultures is not recommended (max 1 week @ 4 °C).
Table 1. Recommended recipes for reagents used.
All the exact recipes of the reagents used in this protocol are outlined here. Specific companies where reagents were purchased are also available in the Table of Materials. Many different sources of chemicals were tested, and those listed in the Table of Materials are those that exhibited the most robust and reproducible results.
| Reagent | Recipe |
|---|---|
| Lysogeny Broth (LB) | In this protocol, commercial LB was used (see materials), but all standard LB home-made recipes using Bacto-tryptone, yeast extract, and NaCl are sufficient. |
| Nematode Growth Media (NGM) | 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl |
| Bleach solution | 1.8% (v/v) sodium hypochlorite, 0.375 M KOH |
| M9 solution | 22 mM KH2PO4 monobasic, 42.3 mM Na2HPO4, 85.6 mM NaCl, 1 mM MgSO4 |
| NGM RNAi plates | 1 mM CaCl2, 5 μ/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μ/mL carbenicillin/ampicillin. Store at 4 ° C in dark for up to 3 months |
| Tetracycline | 10 mg/mL stock solution (500x) in 100% ethanol. Store at −20 °C |
| Carbenicillin | 100 mg/mL stock solution (1000x) in water. Store at 4 °C for up to 6 months or −20 °C for long-term storage |
| Sodium azide | 1 M stock (~6.5%) stock solution of sodium azide in water. Store in the dark at 4 °C. This is a 10x solution and is diluted to a 100 mM working stock for most imaging experiments |
| Tunicamycin | 2.5 mg/mL stock solution in 100% DMSO. Store at −80 °C for long-term storage. This is a 100x solution (25 ng/μL working solution) |
| Antimycin A | 15 mM antimycin A stock solution in 100% DMSO. Store at −20 °C. This is a 5000x solution (3 μM working stock) |
| Paraquat | 50 μM solution in water – should be prepared fresh |
| Tert-butyl hydroperoxide (TBHP) | 7.7 M solution in water. This is a 3850x solution (2 mM working stock) |
| NGM RNAi + DMSO0.2 (control for antimycin A) | 1 mM CaCl2, 5 μ/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μ/mL carbenicillin/ampicillin, 0.2% DMSO |
| NGM RNAi + antimycin A | 1 mM CaCl2, 5 μ/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μ/mL carbenicillin/ampicillin, 0.2% DMSO, 3 mM antimycin A |
| NGM RNAi DMSO (control for tunicamycin) | 1 mM CaCl2, 5 μ/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μ/mL carbenicillin/ampicillin; 1% DMSO |
| NGM RNAi TM | 1 mM CaCl2, 5 μ/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μ/mL carbenicillin/ampicillin; 1% DMSO, 25 ng/μL tunicamycin |
| IPTG | 1 M solution in water. |
2. It is recommended to seed a volume of ~100-200 μL of saturated OP50 culture onto a 60 mm NGM plate (see Table 1) for maintenance of worms and 1 mL of saturated OP50 culture onto a 100 mm plate for expanding animals for experimentation.
3. Let plates dry overnight on benchtop. Note: 100mm plates may need more time to dry, especially if using non-vented plates. It is recommended to store all C. elegans plates in air-tight containers stored at 4 °C. It is not recommended to use plates past 6 months old, as desiccation of plates will occur, which will change animal physiology on plates due to different osmotic pressure and stiffness of plates.
4. For standard maintenance, it is recommended to move 10-15 young animals (eggs, L1, or L2 stages) onto a 60 mm plate, although more can be moved if dealing with mutants or transgenic animals with lower fecundity. For animals with developmental or fecundity defects, it is recommended to chunk a more variable pool of animals to prevent loss of the stock. If kept at 15 °C, animals should be moved every week. For 20 °C, animals should be moved every 3-4 days. A fresh thaw of animals should be performed every 25-30 passages (~ 6 months if animals are moved once a week and kept at 15 °C)
5. For expansion, a full 60mm plate can be chunked onto 100 mm plates for expansion. As a frame of reference: animals with wild-type fecundity can have a full 60mm plate cut into 4ths-6ths and chunked onto a large plate at 20 °C for 2 days or 15 °C for 3 days to create a full 100 mm plate without reaching starvation.
1.2. Staging/synchronization of worms using bleaching.
1.2.1. Bleaching protocol to synchronize worms
1. Wash gravid worms (adults full of eggs) off agar plates using M9 (see Table 1). It is recommended to start from a non-synchronized population of worms (i.e. chunked worms from 1.1 step 5 above) to bleach for experiments, as significant genetic drift may occur upon successive rounds of synchronization via bleaching.
2. Move worm/M9 mixture into a 15 mL conical tube using a glass pipette - several plates of worms can be collected into a single 15 mL conical tube.
3. Spin animals down for 30 seconds at 1,000 x g.
4. Aspirate M9 supernatant. It is unnecessary to wash off residual bacteria unless there is an egregious amount of contamination or large clumps of bacteria. If this is the case, we recommend several washes with M9.
5. Prepare a fresh stock of bleaching solution (see Table 1). Bleaching solution can be kept for several days at 4 °C, but we recommend freshly made bleaching solution as inefficient bleaching can result in uneven bleaching, which will cause damage to eggs before eliminating all worm carcasses.
6. Add 2-10 mL of bleaching solution to the animals (~1 mL of bleach solution for every ~0.1 mL of animal pellet).
7. Invert the bleach and worm mixture for ~5 minutes (do not exceed 10 minutes; note that bleaching times may vary and should be titrated specifically for each lab). Vigorous shaking will help dissolve worm carcasses faster and is recommended for optimal preservation of eggs.
8. Periodically look under a dissection microscope or put the conical tube to a light to observe when the adult worm carcasses have fully dissolved and only eggs remain in the tube.
9. Pellet the eggs by spinning at 1,000 x g for 30 sec and then aspirate the supernatant. Eggs can be centrifuged faster than worms without disrupting their integrity, so if unsure of centrifuge speed, eggs can be spun down at up to 2,500 x g without affecting their physiology.
10. Add M9 up to 15 mL and invert the tube to clear bleach off of eggs. Repeat this washing process 2-3 more times to remove bleach.
11. Pipet egg/M9 mix onto plates and grow at 15-20 °C for experimentation. To get a measure of how many worms to use, approximate egg counts can be performed by pipetting 5 microliters of egg mixture onto an agar plate or slide and dividing the egg count by 5 to determine the number of eggs per 1 μL of volume. Averaging of 3 independent counts may improve accuracy. To avoid starvation, a table of recommended egg counts per plate is available in Table 2.
Table 2. Recommended number of animals to plate post-synchronization to avoid starvation.
To avoid starvation, we recommend plating a specific number of animals per condition. Since OP50 grows denser than HT115, more animals can be plated. All numbers listed here are the guidelines used in our lab, and numbers may be slightly different due to several variables and differences between laboratory conditions. Therefore, or recommended numbers are on the lower side in bold font. Max numbers are what we could plate in optimal conditions in our lab without reaching starvation, but it is not recommended to use these values without first titrating your conditions. All numbers are determined with the assumption that bacteria are seeded onto plates and allowed to grow for ~24 hours at ambient temperature (~22 °C) on plates prior to worms being placed on them. Although there is no major difference in starvation rates when plating eggs or L1s, we recommend plating ~10% higher numbers when plating eggs, since not all eggs will hatch post-bleaching.
| Plate Size |
Bacteria Type |
# of animals to reach day 1 adulthood |
# of animals to reach L4 stage |
|---|---|---|---|
| 60 mm | OP50 | 100-150 | 150-300 |
| 60 mm | HT115 | 70-100 | 120-200 |
| 100 mm | OP50 | 600-1000 | 1500-2000 |
| 100 mm | HT115 | 350-600 | 700-1300 |
12. Animals can also be L1 arrested for tighter temporal synchronization by placing egg/M9 mix in a rotator at 20 °C for up to 24 hours. For wild-type animals, no defects in animal physiology were detected when L1 arresting for up to 48 hours. However, mutants that are sensitive to starvation (e.g. lysosome or autophagy mutants) do very poorly with L1 arresting, and thus it is not recommended to perform this synchronization method for mutants that are known to be sensitive to starvation. If tighter synchronization is required for animals that cannot be L1 arrested, please use the egg-lay method described in 1.2.2.
1.2.2. Egg-lay protocol for synchronizing worms
As an alternative method to bleaching, an egg-lay assay can be performed. Egg-lay is used when bleaching of animals does not provide a close enough synchronization, as eggs within the egg sac of adult animals can be as different as 8-12 hours apart. For experimental paradigms where it is critical for animals to be as closely staged as possible, but where L1 arresting is not possible (e.g. in starvation mutants), egg-lay assays are recommended. However, it should be noted that due to the labor involved in the egg-lay protocol, it is less feasible to perform high-scale experiments.
1. Place 4-12 gravid adults (see note in step 2 below) onto a standard OP50 or HT115-seeded NGM plate (see section 1.1 above for recommendations on bacterial strains). Depending on the scale of experiments, multiple plates can be used. Be sure to carefully document how many animals are on each plate for step 3 below.
2. Place animals at desired temperature for experiments (15-20 °C) for 4-8 hours. The number of hours animals are left on the plate will determine how closely synchronized the first egg laid and the last egg laid will be, so the timing can be adjusted as needed. Since shorter incubation times will mean each animal has less time to lay eggs, more animals should be placed onto plates to ensure enough eggs are laid. While the actual egg-laying rate is not fully normalized due to the tendency of animals to go through short bursts of egg-laying rather than a normalized rate of egg-lay, the average rate of eggs laid per animal can be estimated at ~5 eggs/hour for animals exhibiting wild-type fecundity19. When trying to grow animals to day 1 adult stage, it is recommended to time your egg-lay to have <100 eggs per plate to avoid starvation (refer to Table 2 for more details on recommended animals per plate).
3. Remove adult animals from plates. Ensure that all adult animals are removed from plates, as animals will continue to lay eggs and result in an unsynchronized population and/or starvation of the plate.
2. Imaging of transcriptional reporters using fluorescent microscopy
2.1. Growth conditions of worms for imaging of transcriptional reporters
2.1.1. Growth of worms.
1. Inoculate bacterial culture of HT115 harboring pL4440 RNAi plasmid (EV) and/or carrying an RNAi cassette against desired target gene(s) into LB media supplemented with 100 μg/mL carbenicillin and 20 μg/mL tetracycline.
2. Grow culture overnight (~16 hours) to saturation in a shaking 37 °C incubator.
3. Spot 60 mm NGM RNAi plates with 200 μL and 100 mm NGM RNAi plates with 1000 μL of saturated bacterial culture. Let dry in ambient temperature (~22 °C) overnight in the dark (covered loosely with aluminum foil).
4. Place synchronized population of transgenic worms carrying fluorescent reporters (see Table 3 for complete list) onto NGM RNAi plates seeded with bacteria of choice.
Table 3. Transcriptional reporters for assessing activation of cellular stress responses.
The strains listed here are all available through CGC or through special requests to laboratories for use in both qualitative and quantitative imaging methods described in this manuscript. These strains are all derived from the Bristol N2 background. Recommended methods to apply stress to activate the reporters are also provided. All reporters, with the exception of sod-3p::GFP45 and T24B8.5p::GFP46 are described in the text.
| Strain name |
Transgene | Purpose | Recommended application(s) of stress |
Source |
|---|---|---|---|---|
| SJ4005 | hsp-4p::GFP | UPRER | 25 μ/mL tunicamycin | CGC |
| SJ4100 | hsp-6p::GFP | UPRMT | 3 μM antimycin A; RNAi against ETC or mitochondrial ribosome |
CGC |
| SJ4058 | hsp-60p::GFP | UPRMT | 3 μM antimycin A; RNAi against ETC or mitochondrial ribosome |
CGC |
| CL2070 | hsp-16.2p::GFP | heat-shock response | 34 °C 2 hours | CGC |
| AM446 | hsp-70p::GFP | heat-shock response | 34 °C 2 hours | Morimot o Lab |
| CL2166 | gst-4p::GFP | OxSR | 50 mM paraquat; 2 mM tert-butyl hydroperoxide |
CGC |
| CF1553 | sod-3p::GFP | OxSR & insulin signaling | RNAi against daf-2 (insulin receptor) | CGC |
| AU78 | T24B8.5p::GFP | innate immune response | pathogen exposure (e.g. P. aeruginosa) | CGC |
5. Grow up at 15-20 °C to required stages for specific reporters as outlined below.
2.1.2. Considerations for staging of worms for experimentations
1. Most experiments for transcriptional reporters are done at day 1 of adulthood, with the exception of assays that require L4 animals. According to WormAtlas, L4 animals are obtained approximately 2.5 days (~56 hours) of growth at 20° from the egg stage (www.wormatlas.org).
2. For “day 1 adults,” it is recommended to use animals at approximately 3-4 days (~65-96 hours) of growth at 20°C after plating eggs. This wide range is explained as follows: ~65 hours at 20 °C is when animals reach “egg-laying,” which is the true “adulthood” state. 96 hours is when animals enter what WormAtlas describes as “egg-laying maximal,” which is when the adult is a gravid adult and has a full egg sac. This is when animals would be described as being older than day 1 and may start to display differences, and thus the protocols described here are all recommended to start in this “day 1” stage starting as early as 65 hours and as late as 96 hours.
3. For replicates of a single experiment, it is recommended to use a similar time point for more robust reproducibility (e.g. perform all experiments at ~65 hours or ~96 hours after plating eggs.
4. For the assays described here, major differences were not observed when using animals between this ~65-96 hour window.
5. Note: some transgenic animals and mutants display slowed growth rates. For this, two recommendations exist:
a. staggered synchronization: animals can be bleached at different times in order for experimentation to be performed at the same time. This is recommended when technical variability in the assay may be larger than the technical variabilities that may arise from the synchronization assay (e.g. survival assays, which run for long durations may suffer if they are not performed simultaneously).
b. staggered analysis: animals can be bleached at the same time, but the assay itself is performed at different times. This is recommended for very simple assays that don’t have inherent variability (e.g. RNAi-induction of stress responses).
2.2. Protocols for induction of stress responses
2.2.1. Using hsp-4p::GFP as a readout for the activation of the UPRER
2.2.1.1. Inducing ER stress using RNAi
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest onto NGM RNAi plates (see Table 1) as in 2.1. It is recommended to use EV as a control for basal UPRER levels and RNAi knockdown of tag-335 (enzyme for N-linked glycosylation of ER resident proteins; RNAi knockdown has similar effects to tunicamycin treatment) as a positive control for activation of the UPRER under ER stress.
2. Synchronize hsp-4p::GFP reporter animals using methods described in 1.2.
3. Plate eggs onto RNAi plates using criteria recommended in Table 2.
4. Incubate eggs at 20 °C for approximately 3-4 days (~65-96 hours) to perform experiments at day 1 of adulthood. For the UPRER experiments, there are differences in basal fluorescence of the hsp-4::GFP reporter when grown at different temperatures. Therefore, all experiments should be performed at 20°C.
2.2.1.2. Inducing ER stress using a chemical agent
1. Synchronize hsp-4p::GFP reporter animals using methods described in 1.2 and grow at 20 °C until animals reach the L4 stage. Transfer animals to a tube of M9. Allow worms to settle, then remove M9.
2. Dilute tunicamycin to 25 ng/ml in M9 (a 1:40 dilution from 1mg/ml stock). As a control, also dilute an equivalent volume of DMSO in M9.
3. Add 25 ng/ml tunicamycin/M9 or control DMSO/M9 solution to worms (use 400 – 500 ml for a 1.5ml tube). Incubate at 20°C on a rotating platform for 3-4 hours.
4. Allow worms to settle, remove M9/TM solution, and wash with 1 ml M9.
5. Transfer animals to NGM plates or NGM RNAi plates and allow to recover overnight (or ~15-20 hours) and reach Day 1 of adulthood at 20 °C prior to performing fluorescent microscopy (section 2.3). An overnight recovery is performed to allow for a detectable level of GFP to accumulate.
Induction of hsp-4p::GFP can also be performed by moving L4 animals to agar plates containing 25 ng/μL tunicamycin for 16-24 hours. This has a much more robust induction of hsp-4p::GFP due to the longer duration of stress, and thus the dynamic range is much lower than the assay described above.
2.2.3. Using hsp-6p::GFP as a readout for the activation of the UPRMT
2.2.3.1. Inducing mitochondrial stress using RNAi
UPRMT can be activated following the same protocol as 2.2.1.1, except that RNAi knockdown of mitochondrial genes, such as cox-5b/cco-1 (cytochrome c oxidase subunit 5B; knockdown inhibits electron transport chain activity) can be used. For UPRMT activation through perturbations in electron transport chain function, RNAi knockdown needs to be performed during early development20. Therefore, it is recommended to perform RNAi from hatch for these experiments.
2.2.3.2. Inducing mitochondrial stress using a chemical agent
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. We recommend using HT115 bacteria even in experiments not involving RNAi knockdown (see 1.1). Ensure that both NGM RNAi plates (or NGM RNAi + DMSO0.2) and NGM RNAi + antimycin A plates (see Table 1) are both prepared for step 4.
2. Synchronize hsp-6p::GFP or hsp-60p::GFP reporter animals using methods described in 1.2.
3. Plate eggs onto seeded plates of choice using criteria recommended in Table 2. Since antimycin A is dissolved in DMSO, it is recommended to grow the animals on NGM RNAi + DMSO0.2 plates from hatch.
4. Incubate eggs in 20 °C for 2 days (~56 hours) to the L4 stage. Again, animals can be grown at 15 °C for 3 days (~75 hours) instead.
4. Move worms from NGM RNAi + DMSO0.2 plates to NGM RNAi + antimycin A plates or NGM RNAi + DMSO0.2 plates as a control. Worms can be moved manually with a pick for small-scale experiments, but for large-scale experiments, we recommend washing animals with M9, settling with centrifugation, aspirating M9, and then plating to NGM RNAi + antimycin A plates.
5. Incubate worms for an additional ~20 hours and image at day 1 adult (section 2.3).
2.2.4. Using gst-4p::GFP as a readout for the oxidative stress response.
2.2.4.1. Inducing OxSR using RNAi
gst-4p::GFP can also be induced using RNAi-knockdown of wdr-23 (it encodes a negative regulator of skn-1, and thus its knockdown induces OxSR) using the protocol described in 2.2.2.1.
2.2.4.2. Inducing the OxSR using exposure to the chemical oxidant, Tert-butyl hydroperoxide (TBHP)
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. We recommend using HT115 bacteria even in experiments not involving RNAi knockdown (see 1.1).
2. Synchronize gst-4p::GFP reporter animals using methods described in 1.2.
3. Plate eggs onto seeded plates of choice using criteria recommended in Table 2. Be sure to prepare more plates than necessary as the drug treatment protocol results in loss of > 10-20% of animals. For imaging, it is recommended to start with > 100 animals, and for sorting, it is recommended to start with > 1,000 animals.
4. Incubate eggs in 20 °C for 2 days (~56 hours) to the L4 stage. Animals can be grown at 15 °C for 3 days (~75 hours) instead.
5. Wash L4 animals off the plates and split into two 15 mL falcon tubes per condition.
6. Aspirate volume down to at least 1 mL, and add an equal volume of freshly made 2 mM TBHP. It is recommended for the total volume of liquid to be at least 2 mL, as lower volumes can cause significant death of worms when spinning. It is also not recommended to wash prior to drug treatment, as residual bacteria from plates will help to ensure worms to not over-starve during incubation.
7. Incubate worms on the rotator for 4 hours at 20 °C.
8. Wash worms by spinning at 1,000 x g, aspirating M9 + TBHP mix, and replacing with 15 mL M9.
9. Repeat step 8 for a second wash.
10. Plate worms on EV RNAi to recover overnight (~16 hours) at 20 °C. Worms can be recovered on matching RNAi of choice, but no significant differences were seen when recovered on EV RNAi, so this can be done for ease of experimental set-up.
11. Images are taken 16-24 hours after recovery on day 1 adults. Note: An overnight recovery is performed to allow for a detectable level of GFP to accumulate. Without this recovery, there is no detectable GFP signal. If a shorter term recovery is desired, it is possible to use the assay described in 2.2.4.3.
2.2.4.3. Inducing the OxSR using exposure to the chemical oxidant, paraquat (PQ)
1. Perform steps 1-5 as in 2.2.3.2 to get 2 batches of L4 gst-4p::GFP animals in 15 mL falcon tubes per condition.
2. Aspirate volume down to at least 1 mL, and add an equal volume of freshly made 100 μM PQ. Similar to 2.2.4.2, a minimum volume of 2 mL is recommended.
3. Incubate worms on the rotator for 2 hours at 20 °C.
4. Wash worms by spinning at 1,000 x g, aspirating M9 + PQ mix, and replacing with 15 mL M9.
5. Repeat step 4 for a second wash.
6. Plate worms on EV RNAi to recover for 2 hours at 20 °C.
7. Images are taken 2 hours after recovery at L4 stage. Note: 2 hours of recovery was the minimal recovery required to visualize the GFP induction.
2.2.5. Using hsp-16.2p::GFP and hsp-70p::GFP as a readout for HSR activation.
Inducing HSR using exposure to elevated temperatures
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. It is recommended to use HT115 bacteria, even in experiments not involving RNAi knockdown (see 1.1).
2. Synchronize hsp-16.2p::GFP or hsp-70p::GFP reporter animals using methods described in 1.2.
3. Plate eggs onto seeded plates of choice using criteria recommended in Table 2. Be sure to prepare 2x the number of plates necessary as half of the sample will be exposed to elevated temperatures for heat-shock induction, and the other half will serve as a non-heat-shocked control.
4. Incubate eggs at 20 °C for approximately 3-4 days (~65-96 hours) to perform experiments at day 1 of adulthood. It is not recommended to grow worms at 15 °C for heat-shock experiments as there is a minor difference between animals experiencing heat-shock out of 15 °C versus 20 °C.
5. Move experimental groups of animals to a 34 °C incubator for 2 hours. Plates should be placed in the incubator as a single layer (i.e. no stacking of plates) to ensure the fastest and most equal distribution of heat across the plates.
6. Move heat-shocked animals to 20 °C incubator to recover for 2 hours and then image immediately (section 2.3). Animals can be recovered longer if necessary for higher GFP induction. Note: 2 hours of recovery was the minimal recovery required to visualize the GFP induction.
2.3. Imaging set-up using a stereo microscope or low-magnification wide-field/compound microscope
2.3.1. Preparation of worms for fluorescent microscopy.
1. Pipette 5-10 μl of 100 mM sodium azide on top of a standard NGM plate (containing no bacteria). Note: sodium azide concentration can be brought down as low as 10 mM, although the most robust immobilization was observed at 100 mM sodium azide with no detectable effect on fluorescent signal.
2. Under a dissecting microscope, pick 10-20 animals from experimental plates, and transfer into the spot of sodium azide.
3. Animals should cease movement shortly after landing in sodium azide, and sodium azide itself will evaporate within seconds.
4. Once sodium azide has evaporated, line up animals to desired imaging setup. We recommend moving animals side by side with anterior and posterior sides in the same orientation for all animals.
5. Animals should be imaged immediately, although no changes in reporter signal were observed for any of the transcriptional reporters in Table 3 for up to 15 minutes after paralyzing in sodium azide.
2.3.2. Image acquisition using a stereomicroscope
For this protocol, a Leica M205FA microscope equipped with a Leica DFC3000G monochromatic CCD camera, standard Leica GFP filter (ex 395-455, EM 480 LP), and LAS X software was used. Recommended settings for exposure times can be found in Table 4.
Table 4. Recommended settings for fluorescent microscopy and quantification using a large particle biosorter.
This table serves as guideline for recommended exposure times for fluorescent microscopy or PMT values for the large particle biosorter. These will serve as good starting points, but the exposure time and PMT value should be adjusted for every experiment to ensure that no saturation occurs and that fluorescent values are over the detection limit of background signal. If the sample with the brightest signal for an experiment is known (e.g. positive controls for stress induction), those samples can be used to determine the highest exposure time or PMT that can be used without saturating signal. If the brightest samples are not known, then the control can be used and an exposure time or PMT at the center of the dynamic range of your system can be used.
| Transgene | Recommended application(s) of stress |
Exposure time using a Leica M2250FA stereo microscope |
Exposure time using a Revolve ECHO microscope |
PMT values using a Union Biometrica COPAS biosorter |
|---|---|---|---|---|
| hsp-4p::GFP | 25 μ/mL tunicamycin (~4 hours with overnight recovery) | 200 ms | 275 ms | 450 |
| hsp-6p::GFP | 3 μM antimycin A (~16 hours); RNAi against ETC or mitochondrial ribosome (from hatch) |
100 ms | 50 ms | 350 |
| hsp-60p::GFP | 3 μM antimycin A (~16 hours); RNAi against ETC or mitochondrial ribosome (from hatch) |
200 ms | 100 ms | 450 |
| hsp-16.2p::GFP | 34 °C 2 hours | 400 ms | 200 ms | 500 |
| hsp-70p::GFP | 34 °C 2 hours | 400 ms | 300 ms | 500 |
| gst-4p::GFP | 50 mM paraquat (~2 hours); 2 mM tert-butyl hydroperoxide (~4 hours with overnight recovery) |
100 ms | 50 ms | 350 |
| sod-3p::GFP | RNAi against daf-2 (insulin receptor) | 300 ms | 300 ms | 475 |
| T24B8.5p::GFP | pathogen exposure (e.g. P. aeruginosa) | 100 ms | 50 ms | 350 |
1. Launch LAS X program.
2. Start a new project: “Acquisition” tab (on top) => “Open Projects” tab (on left) => Click “Folder” icon to open new project => Right click to rename => “Acquisition” tab => adjust exposure time and zoom to desired settings.
3. Position worm sample under microscope objective and locate correct focal point of worms using the bright-field setting to minimize fluorescent bleaching. The center of the sample is where the line of eggs is clearly visible and not fuzzy. Set exposure time, zoom, focus, and bright-field condensers to desired settings.
4. Acquire an image using the “Capture Image” button.
5. Images should be saved in .lif (Leica Image File) format as this saves all raw images and metadata. A TIFF can also be exported by right clicking the image (or project) and going to “Save As” => “TIFF.” This will store all channels (e.g. bright-field and GFP) with any modifications (e.g. if contrast was adjusted, this will be saved into the TIFF).
2.3.3. Quantitative analysis of fluorescent images
1. If quantitative analysis will be performed, it is recommended to take 3-D images. This is performed by clicking the z-section option labelled with “z” at the top right. Z-sections will be active if this box is red.
2. Optimize z-sections by selecting the range and slice thickness in the bottom left of adjustable options. Whenever possible, use the “system optimized” button for optimal settings.
3. Capture image and store image as described above in 2.3.2. It is recommended to line up worms with spaces between them for easier measurements.
4. Import TIFF images into imaging software of choice – ImageJ is a freely available option and is described in steps 5-X.
5. For ImageJ, from the “analyze” menu, choose “set measurements.” Check the following: area, mean gray value, integrated density, display label.
6. Using the ROI (region of interest) tool, draw an ROI of interest – it is recommended that each worm be measured individually.
7. From the “analyze” menu, choose “measure” or press M.
8. Draw an ROI in the background where there are no worms to measure the background by choosing “measure” in the “analyze” menu or pressing M.
9. Copy or save measurements that appear in the “results” window.
10. Subtract the background integrated density from the integrated density of each measured ROI. The background intensity for an ROI is defined as the product of the background mean gray value and the area of the ROI drawn.
2.3.4. Image acquisition using a Revolve (ECHO) microscope:
For imaging of transcriptional reporters using a compound/wide-field microscope, this protocol uses a Revolve ECHO R4 microscope equipped with an Olympus 4x Plan Fluorite NA 0.13 objective lens, a standard Olympus FITC filter (ex 470/40; em 525/50; DM 560), and an iPad Pro for the camera and to drive the ECHO software. Recommended settings for exposure times can be found in Table 4.
1. Use touchpad to launch the ECHO program.
2. Create a new album and file name.
3. Position plate under the objective lens.
4. Set exposure time and fluorescence intensity by using your baseline (EV/control treatment) and positive control, so that the signal is visible but not saturated.
5. Save a bright-field image and GFP/FITC image.
3. Quantitative measurements of reporters using a large-particle flow cytometer
Growth and preparation of worms for large-particle flow cytometer analysis can follow the same paradigms as section 1 and 2 for preparation of worms for fluorescent imaging, with the exception that a larger number of animals are required. It is recommended to use >500 animals per condition, as some animals are lost during manipulation, not all animals pass the filtering criteria during quantification, and some animals are not properly read by the flow cytometer. Animals ready for sorting should be washed off plates in 5-10 mL of M9 solution into 15 mL conical tubes for subsequent sorting on the flow cytometer.
3.1. Sorting setup using a large particle flow cytometer
3.1.1. Before you turn on the flow cytometer
1. Make sure the Sheath liquid bottle is not empty. Sheath liquid should be prepared from the 250X stock. Sheath fluid should be prepared at least a few hours prior to using the sorter, as there is a small amount of detergent in the sheath fluid, which can cause bubbles that can cause artifacts during acquisition.
2. Ensure that all waste containers are not full.
3.1.2. Turning on the flow cytometer
1. Turn on the Air Compressor – Turn it to “Auto.” Check the pressure gauge – it should be around 30 psi.
2. Turn on the instrument – Use the power switch that is next to the power cord on the left of the instrument.
3. Turn on lasers:
488 light source is usually sufficient for most experiments.
561 light source needs to be used if higher excitation is required for red fluorescence.
4. Open the FlowPilot software, the instrument should make series of clicks switching on the different valves.
5. Turn on the lasers in the software window by clicking “start."
6. Initiate laser in the Argon laser control popup window by hitting “run.” This should cause the laser to turn on and reach around 12 mW. You should also see the 488 light source level go up to around 12.
7. Hit “done” to close the window.
3.1.3. Checks on software before progressing
1. Check pressure gauges – Look at the 4 pressure values displayed in the bottom of the window. The values should be around the original setup (our recommendations are: Sheath 5.5-5.7; Sample 5.7-6.0; Sorter 3.1-3.3; Clean 8.5-8.7). If it looks similar, check the box next to “pressure ok.”
2. Check fluidics – To make sure there are no air bubbles and debris blocking the flow of sheath/sample through the flow cell, click “clean” several times.
3. Check sheath flow rate – For this you need to collect sheath for 60 sec. Switch “off sort,” then switch “on sheath” in the manual controls to start the flow of sheath. Collect in a 15 ml tube for 60 sec, the flow rate should be ~9-10 ml.
3.1.4. Cleaning before use of the flow cytometer
1. Put ~3-5 ml 10% bleach solution into collection ‘cup’ and hit “acquire,” let run for ~ 30 seconds, hit “abort,” remove excess with vacuum.
2. Rinse collection ‘cup’ with deionized water and remove with vacuum, repeat 2X.
3. Put ~3-5 ml COPAS cleaning solution into collection ‘cup’ and hit “acquire,” let run for ~ 30 seconds, hit “abort,” remove excess cleaning solution with vacuum.
4. Rinse collection ‘cup’ with deionized water and remove with vacuum, repeat 2X.
5. Put ~3-5 ml M9 solution into collection ‘cup’ and hit “acquire,” let run for ~ 30 seconds, hit “stop,” remove excess M9 solution with vacuum.
3.2. Running samples on sorter
1. Adjust laser PMT power and size gating based on the condition that causes the brightest activation of your transcriptional reporter of interest. Recommended settings can be found in Table 4.
2. Add prepared worms to ‘cup’.
3. Hit “acquire.”
4. Watch to make sure that all the liquid is not taken up into the machine, this will cause the flow cytometer to take in air and create bubbles in the detector.
5. Hit “abort” when your sample is low and/or you have collected enough animals.
6. Click “setup” => “data storage” => “gated only.” This will save the data only based on the size constraints.
7. Click “store gated” and save your gated data.
8. Click “erase” to erase data.
9. Rinse collection ‘cup’ with deionized water and remove with vacuum, repeat 2X.
10. Repeat steps 1-9 with the rest of your samples.
3.3. Calibration/ Quality Control – If necessary
1. This requires running control 42μM GYR fluorescent particles provided, to calibrate the 488 laser.
2. Press the metal lip on the top of the sample cup to remove the air tube. Unscrew the cap and use syringe to remove liquid from the sample cup.
3. Mix the bottle of control particles well before use and add few ml into the sample cup. Close the cap and put the air back on by pressing it on till it clicks in place.
4. In the software, go to “tools”->”run control particles.”
5. For control particles, reset the PMT values to: GREEN – 325; YELLOW – 365; RED – 575.
6. Click “acquire.” The sheath should turn on followed by sample. Once the beads start to go through the flow cell, you will see the flow rate at the bottom of the screen. Optimally, the flow rate should be between 5-15/sec. If the flow rate is too low or zero - turn the sample valve physically clockwise to increase flow rate. If the flow rate is too high - turn the sample valve physically anti-clockwise to decrease flow rate.
7. Normally, under bead saver mode, 500 beads are read before switching off. The data can be erased and beads re-read.
8. Once the reading is completed, check for clean single peaks for the 5 parameters as well as the CV values. The Coefficient of Variance (CV) should be <15%. Also, make sure the CV values for the three different fluorescent channels are close to each other.
9. Make a record of the QC check, “file” => “save as screen image.”
3.4. Cleaning and Shutdown
1. Use vacuum to remove sample from sample cup and perform steps 1-5 in 3.1.4.
2. Put ~3-5ml deionized water into collection ‘cup’ and hit “acquire,” let run for ~ 30 seconds, hit “abort.” Leave some distilled water behind in the sample cup.
3. Empty the sample recovery cup and the waste bottle.
4. Turn off software: “File”-> “exit”->Turn off without purging.
5. Turn off laser.
6. Turn off instrument.
7. Turn off air compressor.
8. Close the hatch to cover the instrument.
4. Physiological assays to measure stress sensitivity in C. elegans.
4.1. Measurement of ER stress sensitivity using tunicamycin exposure.
Materials:
1. Prepare NGM RNAi DMSO plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. We recommend using HT115 bacteria even in experiments not involving RNAi knockdown (see 1.1). Remember to also seed NGM RNAi TM plates (see Table 1). A sufficient amount of plates should be seeded: plan for ~5-7 sets of NGM RNAi DMSO plates and ~2-3 sets of NGM RNAi TM plates.
2. Synchronize animals of choice using methods described in 1.2.
3. Plate eggs onto NGM RNAi DMSO seeded plates of choice using criteria recommended in Table 2. Be sure to prepare 2x the number of plates necessary as half of the sample will be transferred to NGM RNAi TM plates.
4. Incubate eggs at 20 °C for approximately 3-4 days (~65-96 hours) to day 1 of adulthood.
5. At day 1, lifespans are prepared by transferring animals onto separate plates. To conserve plates (as TM costs are high), use 8 plates of 15 animals per condition, for a total of 120 animals per condition. This allows a manageable number of animals per plate for scoring and allows a sufficient amount of animals for statistical analyses, even with some censorship.
6. For the first 5-7 days, adult animals should be moved away from progeny every day onto a new plate until progeny are no longer visible. During this stage, animals that are bagged, exhibit vulval protrusions/explosions, or crawling up the sides of the plates should be censored, as these are not deaths associated with ER stress sensitivity. Note that TM treatment causes arrest in animals, and thus only 1-2 moves of these animals every 2-3 days is sufficient to minimize the costs associated with producing TM-containing plates. Wild-type animals have an average survival of ~15-17 days on DMSO and 12-14 days on tunicamcyin.
7. After animals have stopped producing progeny, lifespans can be scored every 1-2 days until all animals are scored as dead or censored. TM-treated animals every day during day 6-14 of adulthood for higher resolution.
4.2. Measurement of mitochondrial and oxidative stress sensitivity using exposure to paraquat.
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. We recommend using HT115 bacteria even in experiments not involving RNAi knockdown (see 1.1).
2. Synchronize animals of choice using methods described in 1.2.
3. Plate eggs onto NGM RNAi seeded plates of choice using criteria recommended in Table 1. The assay calls for ~60-100 animals per condition, so prepare accordingly.
4. Incubate eggs at 20 °C for approximately 3-4 days (~65-96 hours) to day 1 of adulthood.
5. Prepare a fresh vial of 100 mM paraquat in M9 solution.
6. Pipette 50-75 μL of M9 + paraquat into as many wells of a flat-bottom 96-well plate as desired. It is generally recommended to have ~8-10 wells per condition containing ~8-10 animals per well. This allows for an easily visible number of animals per well with ~80 animals per strain.
7. Pick 8-10 animals per condition and transfer them into each well containing M9 + paraquat. Use a pick to transfer animals into the wells rather than pipetting to avoid differences in volume and unintended changes in paraquat concentrations.
8. Every 2 hours, score for death of animals in each well. The plates should be tapped gently, which will cause live animals to thrash or bend. Note that it is possible that live animals are sometimes paralyzed long enough to be scored as dead. Therefore, if the number of live animals exceed the number of live animals from a previous time-point, it is likely that animal was alive and should be unscored (e.g. if at hour 4, 2/10 animals are scored as dead, and at hour 6, only 1/10 animals are dead, hour 4 should be rescored as 1/10 animals dead).
4.3. Measurement of heat sensitivity using exposure to elevated temperatures.
1. Prepare plates spotted with RNAi bacteria targeting your gene of interest as in 2.1. We recommend using HT115 bacteria even in experiments not involving RNAi knockdown (see 1.1).
2. Synchronize animals of choice using methods described in 1.2.
3. Plate eggs onto seeded plates of choice using criteria recommended in Table 1. It is recommended to have 60-100 animals per condition for thermotolerance assays. For thermotolerance assays, L1 arrest or egg lay assays should be used for the best synchronization as there is major variability based on age of animals in the assay.
4. Incubate animals at 20 °C for approximately 3-4 days (~65-96 hours) to perform experiments at day 1 of adulthood. It is not recommended to grow worms at 15 °C for heat-shock experiments as there is a minor difference between animals experiencing heat-shock out of 15 °C versus 20 °C.
5. At day 1, animals are prepared by transferring animals onto separate plates. It is generally recommended to have ~10-15 animals per plate with 4-6 plates, for a total of 60 animals. This allows a manageable number of animals per plate for scoring and allows for minimal time for animals to be pulled out of elevated temperatures.
6. Place animals into a 37 °C incubator and score every 2 hours. Start scoring for thermotolerance at 37 °C at hour 5, as little to no death occurs prior to 5 hours. Median thermotolerance is accomplished at ~9 hours, so hour 7, 9, and 11 are critical time points, although due to incubator and lab-to-lab variability, this may need to be titrated per lab. Moreover, any methods to decrease variability will help (e.g. not stacking plates, placing plates in the same area within a single incubator, minimizing time the incubator is opened or closed, taking as few plates out of the incubator at a time to minimize time animals spend outside of 37 °C, etc. A full guide21).
7. Alternative to step 6: animals can be placed at 34 °C instead of 37 °C. Median thermotolerance at 34 °C is at a little over 14 hours in our hands, so time points 12, 14, and 16 are critical in our experience for 34 °C thermotolerance assays.
REPRESENTATIVE RESULTS:
Using transcriptional reporters to measure activation of stress responses
Here, fluorescent transcriptional reporters are used, which serve as robust tools to measure activation of most stress responses in C. elegans. GFP expression is driven under the promoter of canonical targets of master transcriptional regulators involved in responding to compartment-specific stresses. A comprehensive list of commonly used transcriptional reporters is available in Table 3.
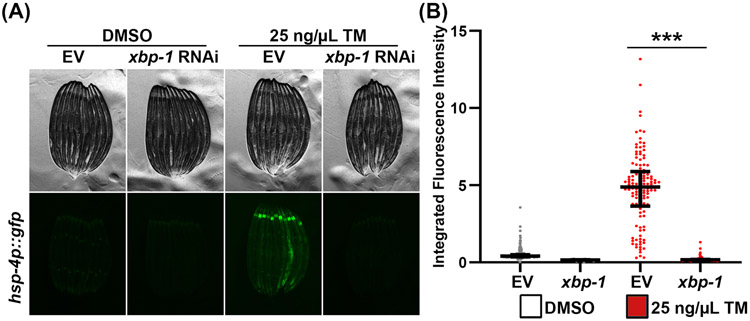
Perturbing ER homeostasis via unfolded or misfolded proteins or lipid bilayer stress causes the activation of the unfolded protein response of the ER (UPRER) to restore ER quality and function. The UPRER consists of 3 distinct branches defined by the transmembrane sensors: inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA (PKR)-like ER kinase (PERK), all of which are conserved in C. elegans7, 22, 23. The most common tool to monitor activation of the UPRER in the nematode, is the transcriptional reporter strain expressing GFP under the control of the hsp-4 promoter (hsp-4p::GFP)7. The gene hsp-4 encodes an orthologue of mammalian Hsp70, HSPA5 (or BiP/Grp78). In times of ER stress when the UPRER is activated, the hsp-4p::GFP reporter strain expresses GFP. This reporter has minimal basal expression in the absence of stress, but exhibits robust GFP expression when animals are exposed to tunicamycin (Figure 1A). These differences can also be quantified using a large-particle flow cytometer (Figure 1B). Moreover, the induction of hsp-4p::GFP under ER stress can be completely suppressed by RNAi-knockdown of xbp-1, as the activation of this transcriptional reporter is dependent on the transcription factor, XBP-112.
Figure 1. Using hsp-4p::GFP as a reporter for UPRER induction.
(A) Representative fluorescent micrographs of hsp-4p::GFP expressing animals grown on control empty vector (EV) or xbp-1 RNAi. Animals were grown on RNAi from hatch until L4 at 20 °C, then treated with 25 ng/μL tunicamycin or 1% DMSO floating in M9 at 20 °C for 4 hours, and recovered on an OP50 plate for 16 hours at 20 °C prior to imaging. Animals were paralyzed in 100 μM sodium azide on an NGM agar plate and imaged using a Leica M205FA stereomicroscope. (B) Quantitative analysis of (A) using a Union Biometrica large particle biosorter. Data is represented as integrated fluorescence intensity across the entire animal where each dot represents a single animal; DMSO control is in grey and tunicamycin treated animals are in red. Central line represents the median, and whiskers represent the interquartile range. n = 123-291 animals per strain. *** = p < 0.001 using non-parametric Mann-Whitney testing.
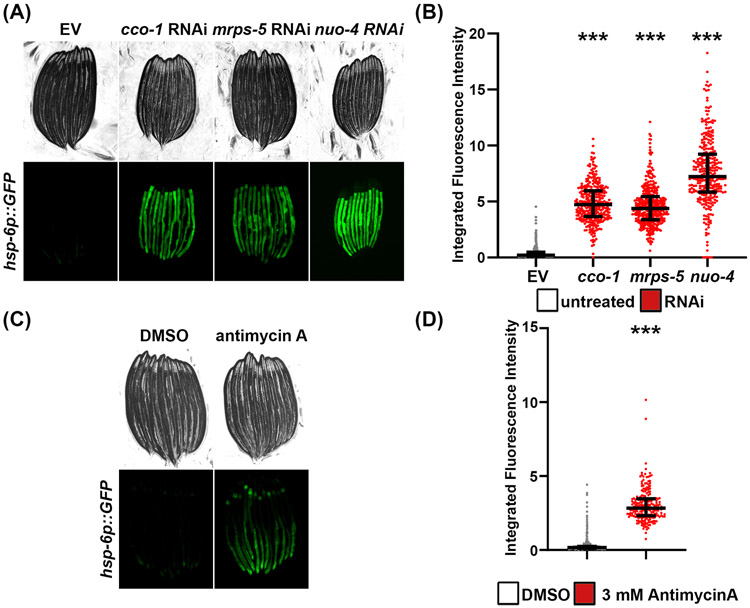
Similar to the UPRER, the mitochondria houses its own protective mechanism against proteotoxic stress. This mechanism, termed the mitochondrial UPR (UPRMT)24, is mainly regulated by the transcription factor, ATFS-1, which fails to enter the mitochondria under stress due to decreased import efficiency, resulting in entry of ATFS-1 into the nucleus25. Interestingly, different perturbations to mitochondrial processes can activate this response, including protein aggregation, knock down of electron transport chain (ETC) complexes subunits, mitochondrial DNA replication stress, and mitochondrial protein translation8, 26. The activation of the UPRMT has been monitored by using worms expressing a transgenic construct in which GFP was placed under the regulation of the promoters of the mitochondrial chaperone genes, hsp-6 and hsp-608. Similar to hsp-4p::GFP animals, hsp-6p::GFP animals exhibit minimal basal signal in the absence of stress. The most robust method to induce the UPRMT is through RNAi-knockdown of the following mitochondrial proteins: cox-5B, the cytochrome c oxidase subunit Vb/COX4 (Complex IV)20, nuo-4, the NADH dehydrogenase protein (Complex I)27, or mrps-5, a mitochondrial ribosomal protein28, activated the hsp-6p::GFP reporter. GFP expression through this reporter is robustly activated and can be easily visualized and quantified under these conditions (Figure 2A-B). The UPRMT can also be triggered through chemical inhibition of the electron transport chain (ETC), such as with antimycin A, which inhibits cytochrome c reductase (complex III). Similar to RNAi-knockdown of ETC components, antimycin A treatment causes a robust induction of hsp-6p::GFP (Figure 2C-D).
Figure 2. Using hsp-6p::GFP as a reporter for UPRMT induction.
(A) Representative fluorescent micrographs of hsp-6p::GFP expressing animals grown on control empty vector (EV), cco-1, mrps-5, or nuo-4 RNAi. Animals were grown on RNAi from hatch and imaged on day 1 of adulthood at 20°C. Animals were paralyzed in 100 μM sodium azide on an NGM agar plate and imaged using a Revolve ECHO R4 compound microscope. (B) Quantitative analysis of (A) using a Union Biometrica large particle biosorter. Data is represented as integrated fluorescence intensity across the entire animal where each dot represents a single animal; EV control is in grey RNAi-treated animals are in red. Central line represents the median, and whiskers represent the interquartile range. n = 303-384 animals per strain. *** = p < 0.001 compared to EV control using non-parametric Mann-Whitney testing. (C) Representative images of hsp-6p::GFP animals treated with DMSO or Antimycin A. Animals were grown from hatch on 0.2% DMSO plates and transferred to plates containing 0.2% DMSO or 3 mM antimycin A for 16 hours prior to imaging on a Revolve ECHO R4 compound microscope. All growth was performed at 20°C. (D) Quantitative analysis of (C) using a large particle biosorter similar to (B). DMSO controls are in great, and Antimycin A-treated animals are in red. n = 495 for DMSO and 219 for Antimycin A. *** = p < 0.001 compared to EV control using non-parametric Mann-Whitney testing.
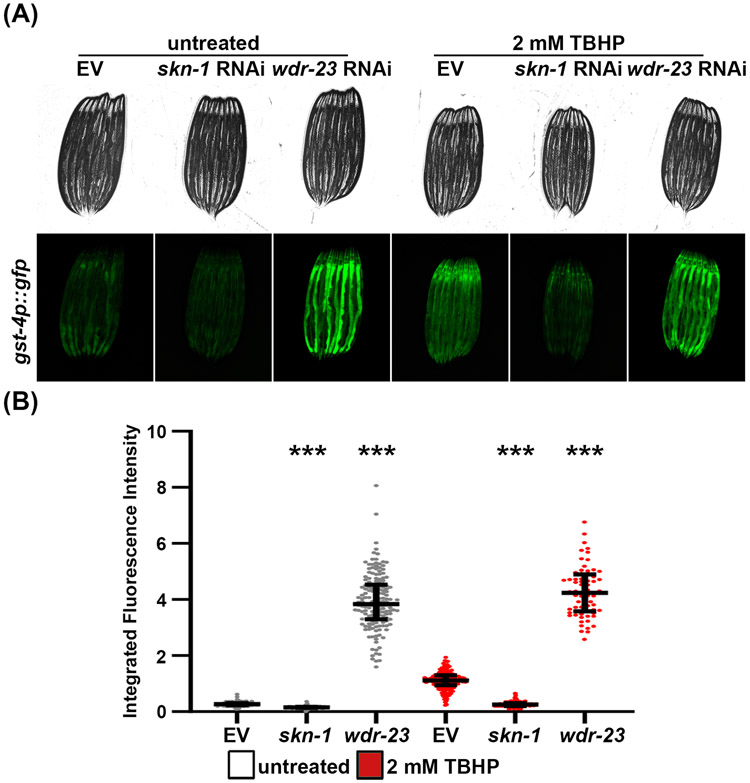
The ability for organisms to sense and respond to oxidative stress is a conserved process present from bacteria to humans29. In C. elegans, the NRF2 homologue, SKN-1, serves as an important transcription factor, which is sensitive to redox changes due to reactive cysteines throughout the protein. SKN-1 serves as one of the transcriptional activator of the OxSR through binding to conserved consensus sequence highly resembling the antioxidant response elements bound by NRF230. In humans, NRF2 is negatively regulated by KEAP1, which is thought to be sensitive to redox changes due to reactive cysteines throughout the protein31. While there is no direct ortholog of KEAP1 in worms, SKN-1 is negatively regulated by the WD-repeat protein, WDR-23, in a manner that is mechanistically distinct from KEAP1/NRF2 inhibition32. Upon oxidative stress, such as tert-butyl hydroperoxide (TBHP), SKN-1 activates detoxification and antioxidant genes such as gst-4, a Glutathione S-Transferase. To measure oxidative stress, GFP expression is placed under the promoter of gst-4, a glutathione S-transferase33. Unlike the other transcriptional regulators presented here, gst-4p::GFP has high basal expression. However, this expression can still be robustly activated under conditions of oxidative stress, which can be performed both genetically and chemically. To genetically induce oxidative stress, we knockdown wdr-23, which encodes a protein that plays a role in proteasomal degradation of SKN-134. wdr-23 knockdown results in robust activation of gst-4p::GFP. Moreover, treatment of worms with the chemical oxidant, TBHP, results in a milder, but still significant, activation of gst-4p::GFP (Figure 3). Both chemical and genetic activation of gst-4p::GFP can be almost completely suppressed by RNAi knockdown of the skn-1, the gene encoding the master transcriptional regulator of the OxSR.
Figure 3. Using gst-4p::GFP as a reporter for the OxSR.
(A) Representative fluorescent micrographs of gst-4p::GFP expressing animals grown on control empty vector (EV), skn-1, or wdr-23 RNAi. Animals were grown on RNAi from hatch until L4 stage at 20 °C. Animals were grown on RNAi from hatch until L4 at 20 °C, then treated with 2 mM TBHP in M9 or only M9 for “untreated” control at 20 °C for 4 hours, and recovered on an EV plate for 16 hours at 20 °C prior to imaging. Animals were paralyzed in 100 μM sodium azide on an NGM agar plate and imaged using a Revolve ECHO R4 compound microscope. (B) Quantitative analysis of (A) using a Union Biometrica large particle biosorter. Data is represented as integrated fluorescence intensity across the entire animal where each dot represents a single animal; untreated control is in grey and TBHP-treated animals are in red. Central line represents the median, and whiskers represent the interquartile range. n = 101-204 animals per strain. *** = p < 0.001 compared to respective EV control using non-parametric Mann-Whitney testing.
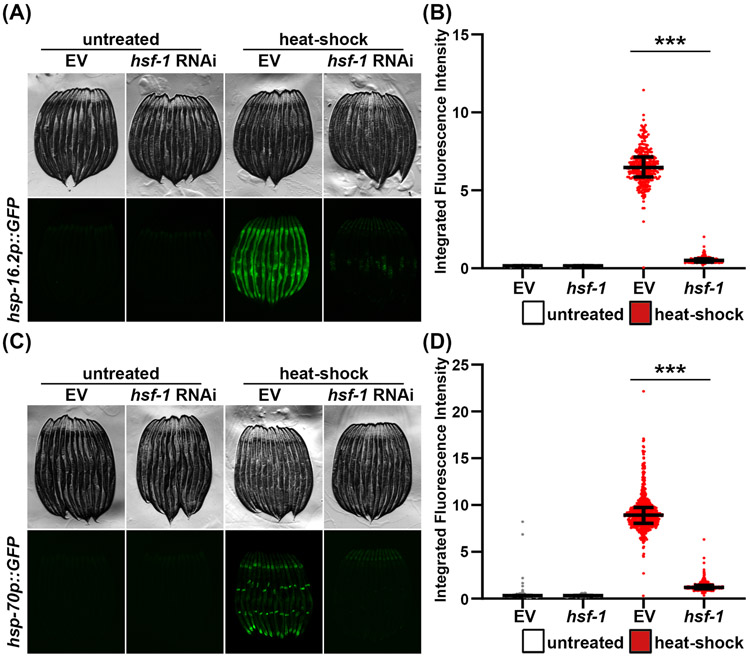
Most cellular proteins are translated in the cytoplasm and reside there, even if only temporarily before being targeted elsewhere. Thus, the cytoplasm hosts a diverse array of chaperones that promote proper protein folding and function, as well as enzymes and proteins responsible for degrading damaged, dysfunctional, or excess proteins. To protect this complex protein landscape of the cytoplasm, the cell has evolved several cytoplasmic stress response pathways, including the heat-shock response (HSR)35, 36. The HSR is a pathway dedicated to promoting protein homeostasis under conditions of heat stress and is modulated by the master transcriptional regulator, HSF-137. Under steady state conditions, HSF-1 is bound by cytoplasmic chaperones, HSP90 and HSP70/40, which keeps it locked in a monomeric, inactive state. Under conditions of heat or similar stress, an increase in misfolded proteins results in titration of chaperones away from HSF-1, allowing it to trimerize and translocate to the nucleus to activate the HSR38, 39. Perhaps the most-studied downstream targets of HSF-1 under HSR activation are the heat-shock proteins (HSPs), such as HSP70, HSP90, DNAJ, and HSP6017, 40. In C. elegans, transcriptional reporters for HSR have been synthesized by driving the expression of GFP under the promoters of canonical HSPs, hsp-16.2 and hsp-709, 41. Like their UPRMT and UPRER counterparts, hsp-16.2p::GFP and hsp-70p::GFP show minimal basal expression in the absence of stress. However, both reporters are robustly induced under conditions of heat stress, which can be easily visualized by microscopy or quantified using a large particle flow cytometer (Figure 4). Both reporters have large dynamic range, and induction is completely dependent on hsf-1, as RNAi-knockdown of hsf-1 fully suppresses induction of hsp-16.2p::GFP and hsp-70p::GFP. While these reporters can be used interchangeably for most situations, there may be differences in expression levels and expression across tissues.
Figure 4. Using hsp16.2p::GFP and hsp-70p::GFP as reporters for the heat-shock response.
(A) Representative fluorescent micrographs of hsp16.2p::GFP expressing animals grown on control empty vector (EV) or hsf-1 RNAi. Animals were grown on RNAi from hatch at 20 °C until day 1. Day 1 animals were either left at 20 °C (untreated) or exposed to 2 hours of heat stress at 34 °C, then recovered for 2 hours at 20 °C. Animals were paralyzed in 100 μM sodium azide on an NGM agar plate and imaged using a Leica M205FA stereomicroscope. (B) Quantitative analysis of (A) using a Union Biometrica large particle biosorter. Data is represented as integrated fluorescence intensity across the entire animal where each dot represents a single animal; untreated control is in grey and heat-shocked animals are in red. Central line represents the median, and whiskers represent the interquartile range. n = 320-364 animals per strain. *** = p < 0.001 using non-parametric Mann-Whitney testing. (C) Representative fluorescent micrographs of hsp-70p::GFP expressing animals grown on control EV and hsf-1 RNAi and treated as described in (A). (D) Quantitative analysis of (C) as described in (B). n = 773-941 animals per strain.
Physiological assays to measure stress sensitivity in C. elegans.
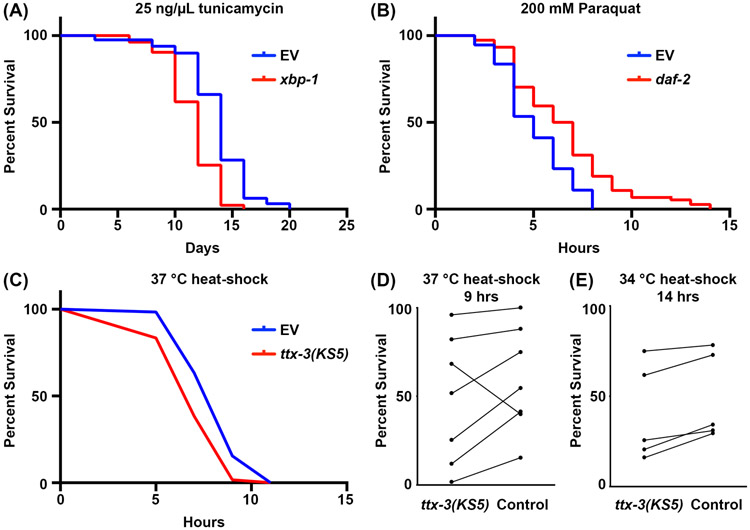
C. elegans are a great model organism to measure stress sensitivity due to the low cost in maintenance and experimentation and ease of genome editing or genetic knockdown using RNAi, which provides the capacity to perform large-scale experiments in a whole organism. To assay stress tolerance to ER stress, we expose C. elegans to the chemical agent, tunicamycin, which causes accumulation of damaged proteins in the ER by blocking N-linked glycosylation10. Animals are exposed to tunicamycin post-development, as the drug causes developmental defects. When exposed to tunicamycin, adult worms exhibit a marked decline in lifespan. Moreover, knockdown of the gene, xbp-1, which encodes one of the primary transcription factors involved in UPRER induction, results in a significant increase in sensitivity to tunicamycin (Figure 5A)12. Thus, this serves as a robust assay to measure ER stress sensitivity in adult worms.
Figure 5. Physiological survival assays under stress in C. elegans.
(A) Lifespans of nematodes grown on 1% DMSO containing 25 ng/μL tunicamycin (TM) plates. Animals were grown on 1% DMSO plates from hatch until day 1, and transferred to respective TM plates at day 1. Animals were kept on control empty vector (EV) or xbp-1 RNAi from hatch until the end of the assay at 20 °C. Adult animals are manually moved away from progeny every day until ~ day 7-8 when progeny were no longer detected, then scored every 2 days until all animals were recorded as dead or censored. Animals with bagging, vulval protrusions/explosions, or those that crawled up the sides of plates were considered censored. (B) Survival curve of nematodes in 100 mM paraquat (PQ) dissolved in M9 solution. Animals were grown on EV or daf-2 RNAi from hatch until day 1 of adulthood at 20 °C. Animals were placed into 50 μL of M9 + PQ solution in a 96 well-plate at 20 °C and visualized every 2 hours until all animals were motionless. (C) Survival curve of nematodes at 37 °C. Wild-type (N2), ttx-3(KS5), and sur-5p::hsf-1 animals were grown on EV plates from hatch until day 1 at 20 °C. At day 1, animals were moved to 37 °C and scored every 2 hours until all animals were scored as dead or censored. (D) Pooled data of all thermotolerance assays performed at 37 °C. Data are represented as percent alive at hour 9 of a thermotolerance assay, with each line representing a matched experiment performed on the same day. (E) Pooled data of all thermotolerance assays performed at 34 °C. Data are represented as percent alive at hour 14 of a thermotolerance assay, with each line representing a matched experiment performed on the same day. All statistics for A-C were performed using Log-Rank (Mantel-Cox) testing and can be found in Table 4.
To measure oxidative stress and mitochondrial stress, we expose animals to the chemical agent, paraquat. Paraquat causes mitochondrial stress by synthesis of ROS within the mitochondrial matrix, which can then be converted into hydrogen peroxide and diffuse out of the mitochondria to cause whole-cell oxidative damage13. Similar to ER stress assays, we expose animals to paraquat at adulthood. However, we perform paraquat assays in liquid to reduce cost and manual labor and agar plate based assays would be difficult for most labs. Here, we show that animals exposed to paraquat in liquid show median survival of approximately 5 hours (Fig. 5B). Moreover, knockdown of the insulin receptor, daf-2, results in an increased resistance to paraquat as activation of DAF-16/FOXO results in increased expression of involved in clearance of ROS, such as sod-342, 43. Paraquat survival assays are short, lasting up to 14 hours, and thus serve as an efficient method to interrogate mitochondrial and oxidative stress responses.
Finally, survival at elevated temperatures is used to interrogate the physiological response to heat stress. These assays can be performed both in liquid or solid agar, and there exist numerous different protocols outlined21. It is recommended to standardize a single assay in the lab to decrease variability, which is exceptionally high in this assay. Thermotolerance should be performed in day 1 adult animals on standard agar plates, either at 34 °C or 37 °C. At 37 °C, a majority of death occurs between 7-11 hours, making this a simple single-day assay, whereas 12-16 hour experiments at 34 °C are most easily performed overnight (Figure 5C-E). Mutation in the gene, ttx-3, results in failure of specification of the AIY interneurons responsible for the thermosensory neural circuit, and causes a significant increase in thermosensitivity44. While thermotolerance data can be plotted as a survival curve (Figure 5C), these assays should be performed at least 4-6 times and all replicates should be plotted against each other (Figure 5D-E), as thermotolerance shows incredibly high variability in comparison to other stress assays. This is due to the many caveats that exist in setting up these experiments, including variability in strains of interest, unequal cycling of air in incubators, uneven agar plates, etc.21. At 34 °C, median survival occurs at approximately 14 hours, and similar to 37 °C, ttx-3 mutants exhibit decreased survival at 34 °C (Figure 5E).
DISCUSSION:
Here, methods to interrogate cellular stress responses in C. elegans, using fluorescent transcriptional reporters and physiological stress survival assays are described. The reporters all utilize GFP expression driven under the promoter of a downstream transcriptional target of the transcription factors involved in mounting cellular stress responses. The use of hsp-4p::GFP modulated by XBP-1s-mediated UPRER, hsp-6p::GFP controlled by ATFS-1-mediated UPRMT, gst-4p::GFP under SKN-1-mediated OxSR, and hsp16.2p::GFP and hsp-70p::GFP under HSF-1-mediated heat-shock response are explained. Other standardized transcriptional reporters can be found in Table 3. All the transcriptional reporters presented here have a wide dynamic range and can be robustly activated by applying stress either through genetic perturbations or exposure to stress-inducing chemicals. Moreover, these reporters can all be suppressed by knockdown of the transcription factors upstream of the promoters employed. Finally, each transcriptional reporter is paired to a specific physiological stress survival assay to provide a physiological readout of the impact of either activating or repressing a specific stress response.
To successfully employ the use of transcriptional reporters, it is essential to determine the dynamic range of each reporter. Due to major lab-to-lab variability caused by differences in media, agar, ambient environment, etc., it is recommended to titrate each drug or stress induction paradigm for concentrations and timing using our recommendations as a baseline. Next, it is critical to ensure the animals are healthy and properly synchronized. Animals that have experienced some sort of stress (e.g. starvation, long-term exposure to light, exposure to elevated temperature, etc.) should be recovered for several generations prior to experimentation. Proper synchronization can be achieved by using the methods outlined in 1.2, which is essential as several stress responses have different levels of activation during the aging process. Finally, imaging protocols are essential to standardize, as there are various things that can affect image and data quality. For example, duration of animals in sodium azide should be minimized, as this causes stress to animals and can affect reporter signal. Moreover, microscope and biosorter specifications should be properly set to maximize signal-to-noise ratio and dynamic range, without causing saturation. Saturated pixels can cause major issues in quantitative analysis of samples, as maximum fluorescent signal can be severely underestimated.
While the transcriptional reporters described here provide a robust and efficient means to measure activation of stress responses, it is critical to understand that it is a single gene target of a known transcription factor. Therefore, while it serves as a reliable method for large-scale screens or first-pass tests of strains of interest, proper validations should be performed. We recommend performing qPCR to measure several canonical target genes activated upon induction of each stress response being assayed. A list of suggested gene targets can be found in Table 6. In addition, transcriptome profiling through RNA-seq is another alternative for a broader look at effects on multiple transcriptional targets at once. Of particular note, the imaging of fluorescent reporters provides spatial information regarding tissues that are affected by the perturbations. Such information cannot be obtained from qRT-PCR or RNA-seq, as it uses whole-worm extracts, except by scRNA-seq, FISH, and tissue-specific RNAseq protocols47. Finally, these stress reporters are generally characterized as being specific to their stress response machinery. However, it is important to remember that not all stress response paradigms are unique and distinct. For example, ER stress can activate OxSR and vice versa48, and overlaps between heat-shock response and ER stress responses have been commonly found49. These are just a few of the many examples in the literature of cross-communication and overlap between stress responses, and thus it is important to understand that all assays should also be tested for specificity to derive final conclusions.
Table 6.
Recommended gene targets and primer pairs for measuring transcriptional upregulation of stress response genes.
| Stress Response |
Target Gene | Forward Primer | Reverse Primer |
|---|---|---|---|
| UPRER | hsp-3 | TCGCTGGATTGAACGTTGTTCG | GTTGCGTTCTCCGTCCTTCTTG |
| UPRER | hsp-4 | GAACAACCTACTCGTGCGTTGG | GAACAACCTACTCGTGCGTTGG |
| UPRER | sel-11 | TTGATCTCCGGAAAACGCAACG | TTGATCTCCGGAAAACGCAACG |
| UPRER | ire-1 | TCCTCAACCGCTCCATCAACAT | TCCTCAACCGCTCCATCAACAT |
| UPRER | xbp-1 | GGACTTCTTCGGCTTCTGGAGT | GGACTTCTTCGGCTTCTGGAGT |
| UPRER | xbp-1 (spliced) | GGTGGATGGAGGGAGAAGATT | GGTGGATGGAGGGAGAAGATT |
| UPRER | crt-1 | GAAGTAATAGCCGAGGGAAGC | GAAGTAATAGCCGAGGGAAGC |
| UPRER | T14G8.3 | CACCTCCATCAACAACAACAT | CACCTCCATCAACAACAACAT |
| HSR | hsp-17 | TCGTTTTCCACCATTCTCCCCA | TGTTTGATCGGCCCAGTATGGT |
| HSR | hsp-70 | TGTTTGATCGGCCCAGTATGGT | TTCGCAATGAGAAGGGACGACT |
| HSR | F44E5.4 | TTCGCAATGAGAAGGGACGACT | CGTTGTGCTGCGTCTTCTCTTT |
| HSR | hsp-16.2 | TCCATCTGAGTCTTCTGAGATTGTTA | TGGTTTAAACTGTGAGACGTTGA |
| HSR | hsf-1 | TTTGCATTTTCTCGTCTCTGTC | TCTATTTCCAGCACACCTCGT |
| UPRMT | hsp-6 | GAGATCGTGGAACCGGAAAGGA | CGGCATTCTTTTCGGCTTCCTT |
| UPRMT | hsp-60 | CGGCATTCTTTTCGGCTTCCTT | CGTCGTTGCAGAGCTCAAGAAG |
| UPRMT | ymel-1 | CAAAACCTGATCTCGCTGGG | TTCTCAATGTCGGCTCCAGT |
| UPRMT | clpp-1 | TGATAAGTGCACCAGTGTCCA | TGATTCTGGAGTTCGGGAGA |
| UPRMT | lonp-1 | CGATGATGGCCATTGTGCAG | CGCTTTGAAACATCAATTTCATCCA |
| OxSR | gst-4 | GATGCTCGTGCTCTTGCTG | CCGAATTGTTCTCCATCGAC |
| OxSR | gst-6 | CCGAATTGTTCTCCATCGAC | TTTGGCAGTTGTTGAGGAG |
| OxSR | gst-7 | TTTGGCAGTTGTTGAGGAG | TGGGTAATCTGGACGGTTTG |
| OxSR | gcs-1 | TGGGTAATCTGGACGGTTTG | ATGTTTGCCTCGACAATGTT |
| OxSR | skn-1 | GGACAACAGAATCCCAAAGG | TCAGGACGTCAACAGCAGAC |
| OxSR | sod-3 | GTAACGGGCGATAGCATGAG | GCGAGAGCACATTGATGAC |
| OxSR | ptps-1 | AATCGATTCCTTTGGAGACC | CAATCTACTGCTCGCACTGCTTCAAAGC |
| reference | pmp-3 | TGGCCGGATGATGGTGTCGC | ACGAACAATGCCAAAGGCCAGC |
| reference | Y45F10.4 | CGAGAACCCGCGAAATGTCGGA | CGGTTGCCAGGGAAGATGAGGC |
| reference | sap-49 | TGGCGGATCGTCGTGCTTCC | ACGAGTCTCCTCGTTCGTCCCA |
| reference | tba-1 | TCAACACTGCCATCGCCGCC | TCCAAGCGAGACCAGGCTTCAG |
Another limitation of the methods described are that imaging and quantification through a biosorter has limited throughput. While biosorter quantification can be performed in 96-well plates for higher throughput, it is still limited by the necessity to transfer worms into solution, whereas imaging is limited by the investigator’s capacity to prepare worms and perform microscopy. Therefore, large-scale screens will most likely involve only a visual screening of fluorescent reporter signal, with only hits being imaged and quantified.
An important caveat with using these fluorescent reporters is that activation or suppression of stress responses do not always contribute to physiologically meaningful phenotypes, or may reflect other global effects (e.g. decrease in protein synthesis). Therefore, every transcriptional reporter is paired to a method to assay stress survival. It is recommended to perform tunicamycin survival assays for the UPRER, paraquat survival assays for the UPRMT and the OxSR, and survival in elevated temperatures for the heat-shock response. While these are generally robust, fast, and simple assays, they require intensive manual labor, and thus are severely limited in scalability. Moreover, almost all thermotolerance assays published to date have major variability, making a large number of replicates almost essential21. While the tunicamycin and paraquat survival assays do not suffer from this lack of reproducibility, they have their own challenges, including extensive hands-on labor and duration of the protocol. As new technologies are born in automating lifespan and survival assays, it is likely that these stress survival assays can also become high-throughput50. However, until these automated assays become standard, survival assays are currently limited to validation of physiological consequences in altering dynamics of stress response activity.
Beyond the stress survival assays described here, there are a number of other methods to measure physiology in animals. For example, a commercially available Seahorse XFp Analyzer can allow monitoring of cellular respiration, which provides additional mechanistic insight51. Another alternative to transcriptional reporters is the use of nuclear localization of fluorescently labelled transcription factors. There are numerous variants of this technique, but of particular interest to the methods described here include: HSF-1::GFP for the heat-shock response52, DVE-1::GFP for the UPRMT53, and DAF-16::GFP and SKN-1::GFP for the OxSR54, 55. Finally, direct interrogation of morphology of specific organelles of interest can be performed. For example, mitochondrial morphology can be visualized by utilizing a fluorophore targeted to the mitochondrial matrix using a mitochondrial-localization sequence56. ER morphology can be visualized by utilizing a fluorophore targeted to the ER through a signal-sequence fused to the N-terminus and HDEL fused to the C-terminus57 or GFP fused to an ER membrane protein58. Finally, actin cytoskeletal integrity can be used as a proxy for thermal stress sensitivity downstream of HSF-1. The actin cytoskeleton is regulated by transcriptional targets of HSF-1, specifically during aging and heat-stress, and thus actin organization can be visualized to determine functional readout of HSF-1 and heat-related stress59, 60.
All of the methods described here can be used independently or in combination with each other for a comprehensive analysis of genes or drugs of interest and their impact on stress response. Large-scale screens can be performed using high-throughput transcriptional reporter assays, and secondary screens can be performed using quantitative analyses of these reporters. Once more manageable candidate gene/drug lists are identified, physiological assays can be performed to identify those candidates that have direct impact on whole animal physiology. The other methods suggested above can also be used as validation or further investigation.
Table 5. Statistics for lifespans and stress survival assays.
All sample sizes, statistics, and censorship rates for Figure 5 are available here.
| Corresponding Figure |
Strain, Treatment | Median Lifespan |
# Deaths/# Total |
% change in median lifespan |
p-value (Log- Rank; Mantel- Cox) |
|---|---|---|---|---|---|
| 5A | N2, vector RNAi, 1% DMSO | 22 days | 95/120 | -- | -- |
| N2, xbp-1 RNAi, 1% DMSO | 14 days | 92/120 | −36.4 | < 0.001 | |
| N2, vector RNAi, 25 ng/μL TM | 14 days | 97/120 | −36.4 | < 0.001 | |
| N2, xbp-1 RNAi, 25 ng/μL TM | 12 days | 98/120 | −45.4 | < 0.001 | |
| 5B | N2, vector RNAi, 100 mM PQ | 5 hours | 73/73 | -- | -- |
| N2, daf-2 RNAi, 100 mM PQ | 6.5 hours | 74/74 | 30 | < 0.001 | |
| 5C | N2, vector RNAi, 37 °C | 8 hours | 59/60 | -- | -- |
| ttx-3(KS5), vector RNAi, 37 °C | 7 hours | 60/60 | −12.5 | 0.002 | |
| sur-5p::hsf-1, vector RNAi, 37 °C | 9 hours | 59/60 | 12.5 | 0.012 |
Table of Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| Antimycin A | Sigma-Aldrich | A8674 | for mitochondrial stress |
| Bacto Peptone | Fisher Scientific | DF0118072 | for NGM plates |
| BD Difco granulated agar | VWR | 90000-782 | for NGM plates |
| Calcium chloride dihydrate | VWR | 97061-904 | for NGM plates |
| Carbenicillin | BioPioneer | C0051-25 | for RNAi |
| Cholesterol | Sigma-Aldrich | 57-88-5 | for NGM plates |
| DMSO | Sigma-Aldrich | 472301 | solvent for drugs |
| IPTG dioxane free | Denville Scientific | CI8280-4 | for RNAi |
| LB Broth Miller | Fisher Scientific | BP1426500 | for LB |
| Magnesium sulfate heptahydrate | VWR | EM-MX0070-3 | for NGM plates, M9 |
| Paraquat | Sigma-Aldrich | 36541 | for oxidative/mitochondrial stress |
| Potassium Chloride | Fisher | P217-500 | for bleach soluton |
| Potassium phosphate dibasic | VWR | EM-PX1570-2 | for NGM plates |
| Potassium phosphate monobasic | VWR | EM-PX1565-5 | for M9 |
| Sodium Azide | Sigma-Aldrich | 71289-50G | for imaging |
| Sodium Chloride | EMD Millipore | SX0420-5 | for NGM plates, M9 |
| Sodium phosphate dibasic | VWR | 71003-472 | for M9 |
| Tert-butyl hydroperoxide | Sigma-Aldrich | 458139 | for oxidative stress |
| Tetracycline hydrochloride | Sigma-Aldrich | T7660-5G | for RNAi |
| Tunicamycin | Sigma-Aldrich | T7765-50MG | for ER stress |
| M205FA stereoscope | Leica | 10450040 | equipped with a Leica DFC3000G monochromatic CCD camera, standard Leica GFP filter (ex 395-455, EM 480 LP), and LAS X software |
| Revolve | ECHO | 75990-514 | equipped with an Olympus 4x Plan Fluorite NA 0.13 objective lens, standard Olympus FITC filter (ex 470/40; em 525/50; DM 560), and an iPad Pro for camera and to drive ECHO software |
| COPAS Biosorter | Union Biometrica | 350-5000-000 | equipped with a 488 nm light source. |
| COPAS Cleaning Solution | Union Biometrica | 300-5072-000 | to use with COPAS |
| COPAS Sheath Solution | Union Biometrica | 300-5070-100 | to use with COPAS |
ACKNOWLEDGMENTS:
R.BZ. is supported by the EMBO long term fellowship and The Larry L. Hillblom Foundation. R.H.S is supported by grant 5F32AG032023-02 through the National Institute of Aging (NIA) and the Glenn Foundation for Medical Research Postdoctoral Fellowship. A.F. is supported by grant F32AG051355 through the NIA. H.K.G. is supported by grant DGE1752814 through the National Science Foundation Graduate Research Fellowship Program. M.G.M. is supported by 1F31AG060660-01 through NIA. A.D. is supported by the Thomas and Stacey Siebel Foundation, the Howard Hughes Medical Institute, and 4R01AG042679-04 and 5R01AG055891-02 from NIA, and 5R01ES021667-09 from NIEHS. We thank Larry Joe, Melissa Sanchez, Naame Kelet, and Anel Esquivel for significant technical assistance. We thank the Morimoto lab and the CGC (funded by NIH Office of Research Infrastructure Program P40 OD010440) for strains.
Footnotes
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES:
- 1.Higuchi-Sanabria R, Frankino PA, Paul JW, Tronnes SU, Dillin A A Futile Battle? Protein Quality Control and the Stress of Aging. Developmental Cell. 44 (2), 139–163, doi: 10.1016/j.devcel.2017.12.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner S The genetics of Caenorhabditis elegans. Genetics. 77 (1), 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rual J-F et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Research. 14 (10B), 2162–2168, doi: 10.1101/gr.2505604 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmons L, Court DL, Fire A Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 263 (1–2), 103–112, doi: 10.1016/s0378-1119(00)00579-5 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Reinke SN, Hu X, Sykes BD, Lemire BD Caenorhabditis elegans diet significantly affects metabolic profile, mitochondrial DNA levels, lifespan and brood size. Molecular Genetics and Metabolism. 100 (3), 274–282, doi: 10.1016/j.ymgme.2010.03.013 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Revtovich AV, Lee R, Kirienko NV Interplay between mitochondria and diet mediates pathogen and stress resistance in Caenorhabditis elegans. PLOS Genetics. 15 (3), e1008011, doi: 10.1371/journal.pgen.1008011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calfon M et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 415 (6867), 92–96, doi: 10.1038/415092a (2002). [DOI] [PubMed] [Google Scholar]
- 8.Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. Journal of Cell Science. 117 (Pt 18), 4055–4066, doi: 10.1242/jcs.01275 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Link CD, Cypser JR, Johnson CJ, Johnson TE Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress & Chaperones. 4 (4), 235–242, doi: (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heifetz A, Keenan RW, Elbein AD Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 18 (11), 2186–2192, doi: 10.1021/bi00578a008 (1979). [DOI] [PubMed] [Google Scholar]
- 11.Struwe WB, Hughes BL, Osborn DW, Boudreau ED, Shaw KMD, Warren CE Modeling a congenital disorder of glycosylation type I in C. elegans: a genome-wide RNAi screen for N-glycosylation-dependent loci. Glycobiology. 19 (12), 1554–1562, doi: 10.1093/glycob/cwp136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RC, Dillin A XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 153 (7), 1435–1447, doi: 10.1016/j.cell.2013.05.042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castello PR, Drechsel DA, Patel M Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. The Journal of Biological Chemistry. 282 (19), 14186–14193, doi: 10.1074/jbc.M700827200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberley LW, Buettner GR Role of Superoxide Dismutase in Cancer: A Review. Cancer Research. 39 (4), 1141–1149 (1979). [PubMed] [Google Scholar]
- 15.Senchuk MM, Dues DJ, Van Raamsdonk JM Measuring Oxidative Stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio-protocol. 7 (1), doi: 10.21769/BioProtoc.2086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE Thermotolerance of a long-lived mutant of Caenorhabditis elegans. Journal of Gerontology. 49 (6), B270–276, doi: 10.1093/geronj/49.6.b270 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Labbadia J, Morimoto RI The Biology of Proteostasis in Aging and Disease. Annual Review of Biochemistry. 84 (1), 435–464, doi: 10.1146/annurev-biochem-060614-033955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H-EH, Jung Y, Lee S-JV Survival assays using Caenorhabditis elegans. Molecules and Cells. 40 (2), 90–99, doi: 10.14348/molcells.2017.0017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waggoner LE, Hardaker LA, Golik S, Schafer WR Effect of a Neuropeptide Gene on Behavioral States in Caenorhabditis elegans Egg-Laying. Genetics. 154 (3), 1181–1192 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durieux J, Wolff S, Dillin A The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 144 (1), 79–91, doi: 10.1016/j.cell.2010.12.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zevian SC, Yanowitz JL Methodological Considerations for Heat Shock of the Nematode Caenorhabditis elegans. Methods (San Diego, Calif.). 68 (3), 450–457, doi: 10.1016/j.ymeth.2014.04.015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X, Ellis RE, Sakaki K, Kaufman RJ Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS genetics. 1 (3), e37, doi: 10.1371/journal.pgen.0010037 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frakes AE, Dillin A The UPR(ER): Sensor and Coordinator of Organismal Homeostasis. Molecular Cell. 66 (6), 761–771, doi: 10.1016/j.molcel.2017.05.031 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Martinus RD et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. European Journal of Biochemistry. 240 (1), 98–103 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science (New York, N.Y.). 337 (6094), 587–590, doi: 10.1126/science.1223560 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker BM, Haynes CM Mitochondrial protein quality control during biogenesis and aging. Trends in Biochemical Sciences. 36 (5), 254–261, doi: 10.1016/j.tibs.2011.01.004 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Shore DE, Carr CE, Ruvkun G Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways. PLOS Genetics. 8 (7), e1002792, doi: 10.1371/journal.pgen.1002792 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houtkooper RH et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 497 (7450), 451–457, doi: 10.1038/nature12188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang SM, Schellhorn HE Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Archives of Biochemistry and Biophysics. 525 (2), 161–169, doi: 10.1016/j.abb.2012.02.007 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radical Biology & Medicine. 88 (Pt B), 290–301, doi: 10.1016/j.freeradbiomed.2015.06.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen T, Nioi P, Pickett CB The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of Biological Chemistry. 284 (20), 13291–13295, doi: 10.1074/jbc.R900010200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo JY, Spatola BN, Curran SP WDR23 regulates NRF2 independently of KEAP1. PLOS Genetics. 13 (4), e1006762, doi: 10.1371/journal.pgen.1006762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link C, Johnson C Reporter Transgenes for Study of Oxidant Stress in Caenorhabditis elegans. Methods in enzymology. 353, 497–505, doi: 10.1016/S0076-6879(02)53072-X (2002). [DOI] [PubMed] [Google Scholar]
- 34.Choe KP, Przybysz AJ, Strange K The WD40 Repeat Protein WDR-23 Functions with the CUL4/DDB1 Ubiquitin Ligase To Regulate Nuclear Abundance and Activity of SKN-1 in Caenorhabditis elegans. Molecular and Cellular Biology. 29 (10), 2704–2715, doi: 10.1128/MCB.01811-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velazquez JM, Lindquist S hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 36 (3), 655–662 (1984). [DOI] [PubMed] [Google Scholar]
- 36.Tissières A, Mitchell HK, Tracy UM Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. Journal of Molecular Biology. 84 (3), 389–398 (1974). [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Pastor R, Burchfiel ET, Thiele DJ Regulation of heat shock transcription factors and their roles in physiology and disease. Nature Reviews Molecular Cell Biology. doi: 10.1038/nrm.2017.73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guisbert E, Czyz DM, Richter K, McMullen PD, Morimoto RI Identification of a tissue-selective heat shock response regulatory network. PLoS genetics. 9 (4), e1003466, doi: 10.1371/journal.pgen.1003466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hentze N, Le Breton L, Wiesner J, Kempf G, Mayer MP Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. eLife. 5, doi: 10.7554/eLife.11576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Labbadia J, Morimoto RI Rethinking HSF1 in Stress, Development, and Organismal Health. Trends in Cell Biology. doi: 10.1016/j.tcb.2017.08.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley JF, Morimoto RI Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Molecular Biology of the Cell. 15 (2), 657–664, doi: 10.1091/mbc.E03-07-0532 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senchuk MM et al. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genetics. 14 (3), doi: 10.1371/journal.pgen.1007268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson ST, Bonafè M, Johnson TE daf-16 protects the nematode Caenorhabditis elegans during food deprivation. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 61 (5), 444–460, doi: 10.1093/gerona/61.5.444 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Prahlad V, Cornelius T, Morimoto RI Regulation of the Cellular Heat Shock Response in Caenorhabditis elegans by Thermosensory Neurons. Science. 320 (5877), 811–814, doi: 10.1126/science.1156093 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libina N, Berman JR, Kenyon C Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 115 (4), 489–502, doi: 10.1016/s0092-8674(03)00889-4 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host & Microbe. 6 (4), 321–330, doi: 10.1016/j.chom.2009.09.001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaletsky R et al. Transcriptome analysis of adult Caenorhabditis elegans cells reveals tissue-specific gene and isoform expression. PLoS Genetics. 14 (8), doi: 10.1371/journal.pgen.1007559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glover-Cutter KM, Lin S, Blackwell TK Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf. PLOS Genetics. 9 (9), e1003701, doi: 10.1371/journal.pgen.1003701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Chang A Heat shock response relieves ER stress. The EMBO journal. 27 (7), 1049–1059, doi: 10.1038/emboj.2008.42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stroustrup N, Ulmschneider BE, Nash ZM, López-Moyado IF, Apfeld J, Fontana W The Caenorhabditis elegans Lifespan Machine. Nature Methods. 10 (7), 665–670, doi: 10.1038/nmeth.2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung DTH, Chu S Measurement of Oxidative Stress: Mitochondrial Function Using the Seahorse System. Methods in molecular biology (Clifton, N.J.). 1710, 285–293, doi: 10.1007/978-1-4939-7498-6_22 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Morton EA, Lamitina T Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 12 (1), 112–120, doi: 10.1111/acel.12024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Developmental Cell. 13 (4), 467–480, doi: 10.1016/j.devcel.2007.07.016 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Kwon E-S, Narasimhan SD, Yen K, Tissenbaum HA A new DAF-16 isoform regulates longevity. Nature. 466 (7305), 498–502, doi: 10.1038/nature09184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.An JH et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proceedings of the National Academy of Sciences of the United States of America. 102 (45), 16275–16280, doi: 10.1073/pnas.0508105102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniele JR, Esping DJ, Garcia G, Parsons LS, Arriaga EA, Dillin A High-Throughput Characterization of Region-Specific Mitochondrial Function and Morphology. Scientific Reports. 7 (1), 6749, doi: 10.1038/s41598-017-05152-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniele JR et al. A non-canonical arm of UPRER mediates longevity through ER remodeling and lipophagy. bioRxiv. 471177, doi: 10.1101/471177 (2018). [DOI] [Google Scholar]
- 58.Xu N et al. The FATP1–DGAT2 complex facilitates lipid droplet expansion at the ER–lipid droplet interface. The Journal of Cell Biology. 198 (5), 895–911, doi: 10.1083/jcb.201201139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baird NA et al. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science (New York, N.Y.). 346 (6207), 360–363, doi: 10.1126/science.1253168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higuchi-Sanabria R et al. Spatial regulation of the actin cytoskeleton by HSF-1 during aging. Molecular Biology of the Cell. 29 (21), 2522–2527, doi: 10.1091/mbc.E18-06-0362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]