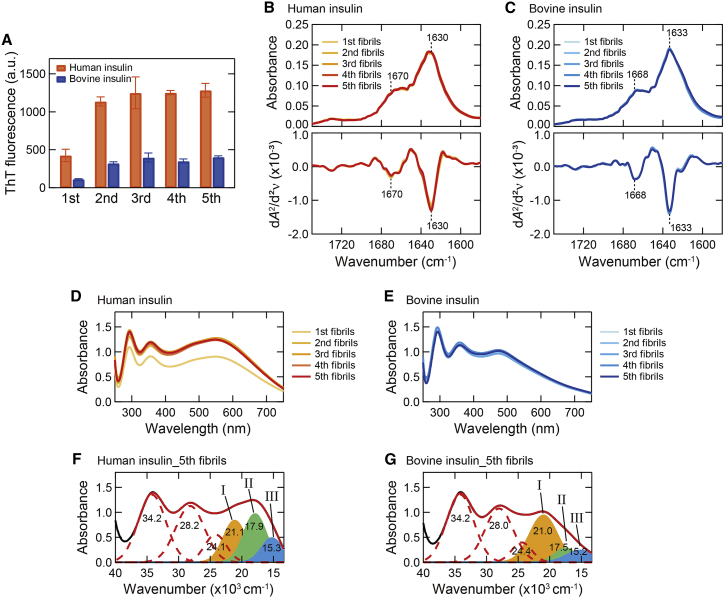
Figure 3.
Self-seeding of human and bovine insulin. (A) ThT fluorescence intensity of the first–fifth amyloid fibrils formed by repeats of self-seeding. The first fibrils correspond to the spontaneously formed amyloid fibrils analyzed in Figs. 1 and 2 and were used as original seeds in this analysis. All samples were assayed in triplicate, and error bars depict standard deviation (SD). (B and C) ATR-FTIR absorption spectra of the first–fifth amyloid fibrils (upper panel) and their second derivatives (lower panel) in amide I region of human (B) and bovine insulin (C). The spectra of the original seeds (i.e., first) are also shown. The positions of two main peaks of β-sheet and β-turn structures are indicated in the graphs. (D and E) UV-Vis absorption spectra of iodine-stained human (D) and bovine insulin fibrils (E) formed by self-seeding. For each absorption spectrum, the spectrum of unstained fibrils was subtracted to obtain net spectrum derived from iodine molecules. (F and G) Deconvolution of UV-Vis absorption spectra of iodine-stained final products (i.e., fifth) of human (F) and bovine insulin fibrils (G) obtained by self-seeding. To see this figure in color, go online.